Effect of hypoxia on the expression of αB-crystallin in head and neck squamous cell carcinoma
- PMID: 24725344
- PMCID: PMC3990244
- DOI: 10.1186/1471-2407-14-252
Effect of hypoxia on the expression of αB-crystallin in head and neck squamous cell carcinoma
Abstract
Background: The presence of hypoxia in head and neck squamous cell carcinoma (HNSCC) is associated with therapeutic resistance and increased risk of metastasis formation. αB-crystallin (HspB5) is a small heat shock protein, which is also associated with metastasis formation in HNSCC. In this study, we investigated whether αB-crystallin protein expression is increased in hypoxic areas of HNSCC biopsies and analyzed whether hypoxia induces αB-crystallin expression in vitro and in this way may confer hypoxic cell survival.
Methods: In 38 HNSCC biopsies, the overlap between immunohistochemically stained αB-crystallin and pimonidazole-adducts (hypoxiamarker) was determined. Moreover, expression levels of αB-crystallin were analyzed in HNSCC cell lines under hypoxia and reoxygenation conditions and after exposure to reactive oxygen species (ROS) and the ROS scavenger N-acetylcysteine (NAC). siRNA-mediated knockdown was used to determine the influence of αB-crystallin on cell survival under hypoxic conditions.
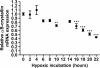
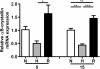
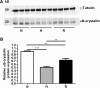
Results: In all biopsies αB-crystallin was more abundantly present in hypoxic areas than in normoxic areas. Remarkably, hypoxia decreased αB-crystallin mRNA expression in the HNSCC cell lines. Only after reoxygenation, a condition that stimulates ROS formation, αB-crystallin expression was increased. αB-crystallin mRNA levels were also increased by extracellular ROS, and NAC abolished the reoxygenation-induced αB-crystallin upregulation. Moreover, it was found that decreased αB-crystallin levels reduced cell survival under hypoxic conditions.
Conclusions: We provide the first evidence that hypoxia stimulates upregulation of αB-crystallin in HNSCC. This upregulation was not caused by the low oxygen pressure, but more likely by ROS formation. The higher expression of αB-crystallin may lead to prolonged survival of these cells under hypoxic conditions.
Figures





Similar articles
-
αB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma.BMC Cancer. 2013 Mar 18;13:128. doi: 10.1186/1471-2407-13-128. BMC Cancer. 2013. PMID: 23506259 Free PMC article.
-
Hypoxia/reoxygenation and TGF-beta increase alphaB-crystallin expression in human optic nerve head astrocytes.Exp Eye Res. 2007 Apr;84(4):694-706. doi: 10.1016/j.exer.2006.12.008. Epub 2006 Dec 15. Exp Eye Res. 2007. PMID: 17261280
-
Alpha-B-crystallin expression in human laryngeal squamous cell carcinoma tissues.Head Neck. 2015 Sep;37(9):1344-8. doi: 10.1002/hed.23746. Epub 2014 Jul 19. Head Neck. 2015. PMID: 24817638
-
αB-Crystallin Phosphorylation: Advances and Problems.Biochemistry (Mosc). 2018 Oct;83(10):1196-1206. doi: 10.1134/S000629791810005X. Biochemistry (Mosc). 2018. PMID: 30472957 Review.
-
AlphaB-crystallin and breast cancer: role and possible therapeutic strategies.Cell Stress Chaperones. 2021 Jan;26(1):19-28. doi: 10.1007/s12192-020-01175-0. Epub 2020 Oct 28. Cell Stress Chaperones. 2021. PMID: 33111264 Free PMC article. Review.
Cited by
-
Nine-gene signature and nomogram for predicting survival in patients with head and neck squamous cell carcinoma.Front Genet. 2022 Aug 24;13:927614. doi: 10.3389/fgene.2022.927614. eCollection 2022. Front Genet. 2022. PMID: 36092911 Free PMC article.
-
Comparative RNA sequencing reveals that HPV16 E6 abrogates the effect of E6*I on ROS metabolism.Sci Rep. 2019 Apr 11;9(1):5938. doi: 10.1038/s41598-019-42393-6. Sci Rep. 2019. PMID: 30976051 Free PMC article.
-
Systems-level effects of ectopic galectin-7 reconstitution in cervical cancer and its microenvironment.BMC Cancer. 2016 Aug 24;16(1):680. doi: 10.1186/s12885-016-2700-8. BMC Cancer. 2016. PMID: 27558259 Free PMC article.
-
Peripherally misfolded proteins exacerbate ischemic stroke-induced neuroinflammation and brain injury.J Neuroinflammation. 2021 Jan 20;18(1):29. doi: 10.1186/s12974-021-02081-7. J Neuroinflammation. 2021. PMID: 33472658 Free PMC article.
-
Progression of the role of CRYAB in signaling pathways and cancers.Onco Targets Ther. 2019 May 30;12:4129-4139. doi: 10.2147/OTT.S201799. eCollection 2019. Onco Targets Ther. 2019. PMID: 31239701 Free PMC article. Review.
References
-
- Mazzone M, Dettori D, Leite De OR, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz De AC, De SF, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

