Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling
- PMID: 24725364
- PMCID: PMC4283731
- DOI: 10.1111/pcmr.12250
Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling
Abstract
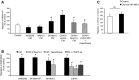
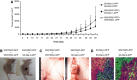
Fibroblast growth factor (FGF2) regulates endothelial and melanoma cell migration. The binding of FGF2 to its receptor requires N-sulfated heparan sulfate (HS) glycosamine. We have previously reported that Epac1, an exchange protein activated by cAMP, increases N-sulfation of HS in melanoma. Therefore, we examined whether Epac1 regulates FGF2-mediated cell-cell communication. Conditioned medium (CM) of melanoma cells with abundant expression of Epac1 increased migration of human umbilical endothelial cells (HUVEC) and melanoma cells with poor expression of Epac1. CM-induced increase in migration was inhibited by antagonizing FGF2, by the removal of HS and by the knockdown of Epac1. In addition, knockdown of Epac1 suppressed the binding of FGF2 to FGF receptor in HUVEC, and in vivo angiogenesis in melanoma. Furthermore, knockdown of Epac1 reduced N-sulfation of HS chains attached to perlecan, a major secreted type of HS proteoglycan that mediates the binding of FGF2 to FGF receptor. These data suggested that Epac1 in melanoma cells regulates melanoma progression via the HS-FGF2-mediated cell-cell communication.
Keywords: Epac; FGF2; angiogenesis; cell-cell communication; heparan sulfate; human umbilical vein endothelial cells; migration; paracrine signaling.
© 2014 The Authors. Pigment Cell & Melanoma Research Published by John Wiley & Sons Ltd.
Figures



References
-
- Baljinnyam E, De Lorenzo MS, Xie LH, Iwatsubo M, Chen S, Goydos JS, Nowycky MC. Iwatsubo K. Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2 + -dependent mechanism. Cancer Res. 2010;70:5607–5617. - PubMed
-
- Baljinnyam E, Umemura M, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS. Iwatsubo K. Epac1 promotes melanoma metastasis via modification of heparan sulfate. Pigment Cell Melanoma Res. 2011;24:680–687. - PubMed
-
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 2006;31:680–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

