Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling
- PMID: 24725405
- PMCID: PMC4001090
- DOI: 10.1016/j.cell.2014.01.066
Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling
Abstract
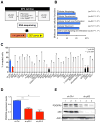
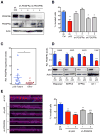
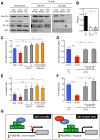
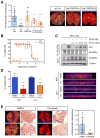
Missense mutations in the p53 tumor suppressor inactivate its antiproliferative properties but can also promote metastasis through a gain-of-function activity. We show that sustained expression of mutant p53 is required to maintain the prometastatic phenotype of a murine model of pancreatic cancer, a highly metastatic disease that frequently displays p53 mutations. Transcriptional profiling and functional screening identified the platelet-derived growth factor receptor b (PDGFRb) as both necessary and sufficient to mediate these effects. Mutant p53 induced PDGFRb through a cell-autonomous mechanism involving inhibition of a p73/NF-Y complex that represses PDGFRb expression in p53-deficient, noninvasive cells. Blocking PDGFRb signaling by RNA interference or by small molecule inhibitors prevented pancreatic cancer cell invasion in vitro and metastasis formation in vivo. Finally, high PDGFRb expression correlates with poor disease-free survival in pancreatic, colon, and ovarian cancer patients, implicating PDGFRb as a prognostic marker and possible target for attenuating metastasis in p53 mutant tumors.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. A Mutantp53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. - PubMed
-
- Ballagi AE, Ishizaki A, Nehlin JO, Funa K. Isolation and characterization of the mouse PDGF beta-receptor promoter. Biochem Biophys Res Commun. 1995;210:165–173. - PubMed
-
- Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. - PubMed
-
- Dai Y. Platelet-derived growth factor receptor tyrosine kinase inhibitors: a review of the recent patent literature. Expert Opin Ther Pat. 2010;20:885–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

