Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response
- PMID: 24725946
- PMCID: PMC7111975
- DOI: 10.1016/j.virol.2014.02.018
Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response
Abstract
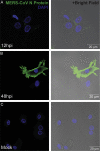
The Middle East respiratory syndrome coronavirus (MERS-CoV) closely resembled severe acute respiratory syndrome coronavirus (SARS-CoV) in disease manifestation as rapidly progressive acute pneumonia with multi-organ dysfunction. Using monocyte-derived-dendritic cells (Mo-DCs), we discovered fundamental discrepancies in the outcome of MERS-CoV- and SARS-CoV-infection. First, MERS-CoV productively infected Mo-DCs while SARS-CoV-infection was abortive. Second, MERS-CoV induced significantly higher levels of IFN-γ, IP-10, IL-12, and RANTES expression than SARS-CoV. Third, MERS-CoV-infection induced higher surface expression of MHC class II (HLA-DR) and the co-stimulatory molecule CD86 than SARS-CoV-infection. Overall, our data suggests that the dendritic cell can serve as an important target of viral replication and a vehicle for dissemination. MERS-CoV-infection in DCs results in the production of a rich combination of cytokines and chemokines, and modulates innate immune response differently from that of SARS-CoV-infection. Our findings may help to explain the apparent discrepancy in the pathogenicity between MERS-CoV and SARS-CoV.
Keywords: Antigen-presentation; Cytokine and chemokine response; MERS-CoV; Pathogenesis; SARS-CoV; Viral replication.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis.J Infect Dis. 2014 May 1;209(9):1331-42. doi: 10.1093/infdis/jit504. Epub 2013 Sep 24. J Infect Dis. 2014. PMID: 24065148 Free PMC article.
-
Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment.J Gen Virol. 2013 Dec;94(Pt 12):2679-2690. doi: 10.1099/vir.0.055533-0. Epub 2013 Sep 28. J Gen Virol. 2013. PMID: 24077366
-
High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus.J Virol. 2015 Apr;89(7):3859-69. doi: 10.1128/JVI.03607-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609809 Free PMC article.
-
Severe acute respiratory syndrome vs. the Middle East respiratory syndrome.Curr Opin Pulm Med. 2014 May;20(3):233-41. doi: 10.1097/MCP.0000000000000046. Curr Opin Pulm Med. 2014. PMID: 24626235 Review.
-
Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis.Virol J. 2015 Dec 22;12:218. doi: 10.1186/s12985-015-0446-6. Virol J. 2015. PMID: 26690369 Free PMC article. Review.
Cited by
-
[Neuroimmunology of COVID-19].Nervenarzt. 2021 Jun;92(6):521-530. doi: 10.1007/s00115-021-01077-1. Epub 2021 Mar 2. Nervenarzt. 2021. PMID: 33651117 Free PMC article. Review. German.
-
What chances do children have against COVID-19? Is the answer hidden within the thymus?Eur J Pediatr. 2021 Mar;180(3):983-986. doi: 10.1007/s00431-020-03841-y. Epub 2020 Oct 13. Eur J Pediatr. 2021. PMID: 33047161 Free PMC article.
-
Pregnancy and COVID-19: management and challenges.Rev Inst Med Trop Sao Paulo. 2020;62:e62. doi: 10.1590/s1678-9946202062062. Epub 2020 Aug 31. Rev Inst Med Trop Sao Paulo. 2020. PMID: 32876296 Free PMC article. Review.
-
Exploiting Signal Joint T Cell Receptor Excision Circle to Investigate the Impact of COVID-19 and Autoimmune Diseases on Age Prediction and Immunosenescence.Biomedicines. 2022 Dec 9;10(12):3193. doi: 10.3390/biomedicines10123193. Biomedicines. 2022. PMID: 36551949 Free PMC article.
-
Chemokines and chemokine receptors during COVID-19 infection.Comput Struct Biotechnol J. 2021;19:976-988. doi: 10.1016/j.csbj.2021.01.034. Epub 2021 Jan 27. Comput Struct Biotechnol J. 2021. PMID: 33558827 Free PMC article. Review.
References
-
- Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E., Badu E.K., Anti P., Agbenyega O., Meyer B., Oppong S., Sarkodie Y.A., Kalko E.K., Lina P.H., Godlevska E.V., Reusken C., Seebens A., Gloza-Rausch F., Vallo P., Tschapka M., Drosten C., Drexler J.F. Human Betacoronavirus 2c EMC/2012-related Viruses in Bats, Ghana and Europe. Emerg. Infect. Dis. 2013;19:456–459. - PMC - PubMed
-
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F., Makhdoom H.Q., Zumla A.I., Memish Z.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. - PMC - PubMed
-
- Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A., Alabdullatif Z.N., Assad M., Almulhim A., Makhdoom H., Madani H., Alhakeem R., Al-Tawfiq J.A., Cotten M., Watson S.J., Kellam P., Zumla A.I., Memish Z.A. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N. Engl. J. Med. 2013;369(5):407–416. - PMC - PubMed
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Chan J.F., Chan K.H., Choi G.K., To K.K., Tse H., Cai J.P., Yeung M.L., Cheng V.C., Chen H., Che X.Y., Lau S.K., Woo P.C., Yuen K.Y. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J. Infect. Dis. 2013;207:1743–1752. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

