Water permeation drives tumor cell migration in confined microenvironments
- PMID: 24726433
- PMCID: PMC4365996
- DOI: 10.1016/j.cell.2014.02.052
Water permeation drives tumor cell migration in confined microenvironments
Abstract
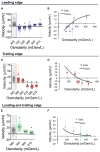
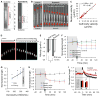
Cell migration is a critical process for diverse (patho)physiological phenomena. Intriguingly, cell migration through physically confined spaces can persist even when typical hallmarks of 2D planar migration, such as actin polymerization and myosin II-mediated contractility, are inhibited. Here, we present an integrated experimental and theoretical approach ("Osmotic Engine Model") and demonstrate that directed water permeation is a major mechanism of cell migration in confined microenvironments. Using microfluidic and imaging techniques along with mathematical modeling, we show that tumor cells confined in a narrow channel establish a polarized distribution of Na+/H+ pumps and aquaporins in the cell membrane, which creates a net inflow of water and ions at the cell leading edge and a net outflow of water and ions at the trailing edge, leading to net cell displacement. Collectively, this study presents an alternate mechanism of cell migration in confinement that depends on cell-volume regulation via water permeation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. - PubMed
-
- Atzberger PJ, Isaacson S, Peskin CS. A microfluidic pumping mechanism driven by non-equilibrium osmotic effects. Physica D. 2009;238:1168–1179.
-
- Beltran AR, Ramirez MA, Carraro-Lacroix LR, Hiraki Y, Reboucas NA, Malnic G. NHE1, NHE2, and NHE4 contribute to regulation of cell pH in T84 colon cancer cells. Pflugers Arch. 2008;455:799–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

