Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis
- PMID: 24726754
- PMCID: PMC4961260
- DOI: 10.1053/j.gastro.2014.04.003
Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis
Abstract
Background & aims: T-cell-mediated biliary injury is a feature of primary sclerosing cholangitis (PSC). We studied the roles of CD28(-) T cells in PSC and their regulation by vitamin D.
Methods: Peripheral and liver-infiltrating mononuclear cells were isolated from blood or fresh liver tissue. We analyzed numbers, phenotypes, functions, and localization patterns of CD28(-) T cells, along with their ability to activate biliary epithelial cells. We measured levels of tumor necrosis factor (TNF)α in liver tissues from patients with PSC and the effects of exposure to active vitamin D (1,25[OH]2D3) on expression of CD28.
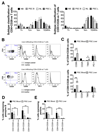
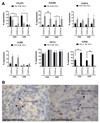
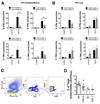
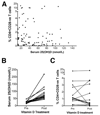
Results: A significantly greater proportion of CD4(+) and CD8(+) T cells that infiltrated liver tissues of patients with PSC were CD28(-), compared with control liver tissue (CD4(+): 30.3% vs 2.5%, P < .0001; and CD8(+): 68.5% vs 31.9%, P < .05). The mean percentage of CD4(+)CD28(-) T cells in liver tissues from patients with PSC was significantly higher than from patients with primary biliary cirrhosis or nonalcoholic steatohepatitis (P < .05). CD28(-) T cells were activated CD69(+)CD45RA(-) C-C chemokine receptor (CCR)7(-) effector memory and perforin(+) granzyme B(+) cytotoxic cells, which express CD11a, CX3CR1, C-X3-C motif receptor 6 (CXCR6), and CCR10-consistent with their infiltration of liver and localization around bile ducts. Compared with CD28(+) T cells, activated CD28(-) T cells produced significantly higher levels of interferon γ and TNFα (P < .05), and induced up-regulation of intercellular cell adhesion molecule-1, HLA-DR, and CD40 by primary epithelial cells (3.6-fold, 1.5-fold, and 1.2-fold, respectively). Liver tissue from patients with PSC contained high levels of TNFα; TNFα down-regulated the expression of CD28 by T cells in vitro (P < .01); this effect was prevented by administration of 1,25(OH)2D3 (P < .05).
Conclusions: Inflammatory CD28(-) T cells accumulate in livers of patients with PSC and localize around bile ducts. The TNFα-rich microenvironment of this tissue promotes inflammation; these effects are reversed by vitamin D in vitro.
Keywords: Autoimmunity; Biliary Epithelial Cells; Immune Regulation; Interferon.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors disclose no conflicts.
Figures



References
-
- Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet. 2013;382:1587–1599. - PubMed
-
- Hashimoto E, Lindor KD, Homburger HA, et al. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993;68:1049–1055. - PubMed
-
- Ponsioen CY, Kuiper H, Ten Kate FJ, et al. Immunohistochemical analysis of inflammation in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1999;11:769–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

