Cell signaling during development of Dictyostelium
- PMID: 24726820
- PMCID: PMC4075484
- DOI: 10.1016/j.ydbio.2014.04.001
Cell signaling during development of Dictyostelium
Abstract
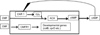
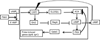
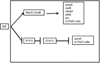
Continuous communication between cells is necessary for development of any multicellular organism and depends on the recognition of secreted signals. A wide range of molecules including proteins, peptides, amino acids, nucleic acids, steroids and polylketides are used as intercellular signals in plants and animals. They are also used for communication in the social ameba Dictyostelium discoideum when the solitary cells aggregate to form multicellular structures. Many of the signals are recognized by surface receptors that are seven-transmembrane proteins coupled to trimeric G proteins, which pass the signal on to components within the cytoplasm. Dictyostelium cells have to judge when sufficient cell density has been reached to warrant transition from growth to differentiation. They have to recognize when exogenous nutrients become limiting, and then synchronously initiate development. A few hours later they signal each other with pulses of cAMP that regulate gene expression as well as direct chemotactic aggregation. They then have to recognize kinship and only continue developing when they are surrounded by close kin. Thereafter, the cells diverge into two specialized cell types, prespore and prestalk cells, that continue to signal each other in complex ways to form well proportioned fruiting bodies. In this way they can proceed through the stages of a dependent sequence in an orderly manner without cells being left out or directed down the wrong path.
Keywords: Dependent sequence; Intercellular communication; Signal transduction.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
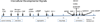









References
-
- Abe H, Uchiyama M, Tanaka Y, Saito H. Structure of discadenine, a spore germination inhibitor from the cellular slime mold Dictyostelium discoideum. Tetrahedron Lett. 1976;42:3807–3810.
-
- Anjard C, Loomis WF. GABA induces terminal differentiation of Dictyostelium through a GABA(B) receptor. Development. 2006;133:2253–2261. - PubMed
-
- Anjard C, Loomis WF. Cytokinins induce sporulation in Dictyostelium. Development. 2008;135:819–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

