A framework for the next generation of risk science
- PMID: 24727499
- PMCID: PMC4123023
- DOI: 10.1289/ehp.1307260
A framework for the next generation of risk science
Abstract
Objectives: In 2011, the U.S. Environmental Protection Agency initiated the NexGen project to develop a new paradigm for the next generation of risk science.
Methods: The NexGen framework was built on three cornerstones: the availability of new data on toxicity pathways made possible by fundamental advances in basic biology and toxicological science, the incorporation of a population health perspective that recognizes that most adverse health outcomes involve multiple determinants, and a renewed focus on new risk assessment methodologies designed to better inform risk management decision making.
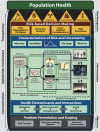
Results: The NexGen framework has three phases. Phase I (objectives) focuses on problem formulation and scoping, taking into account the risk context and the range of available risk management decision-making options. Phase II (risk assessment) seeks to identify critical toxicity pathway perturbations using new toxicity testing tools and technologies, and to better characterize risks and uncertainties using advanced risk assessment methodologies. Phase III (risk management) involves the development of evidence-based population health risk management strategies of a regulatory, economic, advisory, community-based, or technological nature, using sound principles of risk management decision making.
Conclusions: Analysis of a series of case study prototypes indicated that many aspects of the NexGen framework are already beginning to be adopted in practice.
Conflict of interest statement
M.E.A. received support from the Long-Range Research Initiative of the American Chemical Council. The other authors declare they have no actual or potential competing financial interests.
Figures
References
-
- Anastas PT, Sonich-Mullin C, Fried B. Designing science in a crisis: the Deepwater Horizon oil spill. Environ Sci Technol. 2010;44:9250–9251. - PubMed
-
- Andersen ME, Al-Zoughool M, Croteau M, Westphal M, Krewski D. The future of toxicity testing. J Toxicol Environ Health B Crit Rev. 2010;13:163–196. - PubMed
-
- Andersen ME, Krewski D. The vision of toxicity testing in the 21st century: moving from discussion to action. Toxicol Sci. 2010;117:17–24. - PubMed
-
- AXLR8. Overview: What Is AXLR8? 2012. Available: http://axlr8.eu/overview/ [accessed 14 March 2014]
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

