Biosynthesis and deficiencies of glycosylphosphatidylinositol
- PMID: 24727937
- PMCID: PMC4055706
- DOI: 10.2183/pjab.90.130
Biosynthesis and deficiencies of glycosylphosphatidylinositol
Abstract
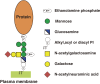
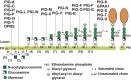
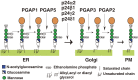
At least 150 different human proteins are anchored to the outer leaflet of the plasma membrane via glycosylphosphatidylinositol (GPI). GPI preassembled in the endoplasmic reticulum is attached to the protein's carboxyl-terminus as a post-translational modification by GPI transamidase. Twenty-two PIG (for Phosphatidyl Inositol Glycan) genes are involved in the biosynthesis and protein-attachment of GPI. After attachment to proteins, both lipid and glycan moieties of GPI are structurally remodeled in the endoplasmic reticulum and Golgi apparatus. Four PGAP (for Post GPI Attachment to Proteins) genes are involved in the remodeling of GPI. GPI-anchor deficiencies caused by somatic and germline mutations in the PIG and PGAP genes have been found and characterized. The characteristics of the 26 PIG and PGAP genes and the GPI deficiencies caused by mutations in these genes are reviewed.
Figures
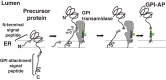
References
-
- Ferguson, M.A.J., Kinoshita, T. and Hart, G.W. (2009) Glycosylphosphatidylinositol anchors. In Essentials of Glycobiology (eds. Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W. and Etzler, M.E.). 2nd ed. Cold Spring Harbor, New York, pp. 143–161. - PubMed
-
- Kinoshita T., Fujita M., Maeda Y. (2008) Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J. Biochem. 144, 287–294 - PubMed
-
- Suzuki K.G., Kasai R.S., Hirosawa K.M., Nemoto Y.L., Ishibashi M., Miwa Y., Fujiwara T.K., Kusumi A. (2012) Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 8, 774–783 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

