Phosphate starvation in fungi induces the replacement of phosphatidylcholine with the phosphorus-free betaine lipid diacylglyceryl-N,N,N-trimethylhomoserine
- PMID: 24728191
- PMCID: PMC4054272
- DOI: 10.1128/EC.00004-14
Phosphate starvation in fungi induces the replacement of phosphatidylcholine with the phosphorus-free betaine lipid diacylglyceryl-N,N,N-trimethylhomoserine
Abstract
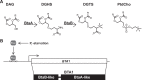
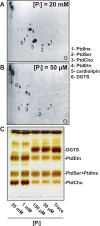
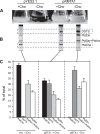
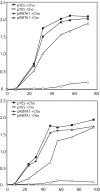
Diacylglyceryl-N,N,N-trimethylhomoserine (DGTS) is a phosphorus-free betaine-lipid analog of phosphatidylcholine (PtdCho) synthesized by many soil bacteria, algae, and nonvascular plants. Synthesis of DGTS and other phosphorus-free lipids in bacteria occurs in response to phosphorus (P) deprivation and results in the replacement of phospholipids by nonphosphorous lipids. The genes encoding DGTS biosynthetic enzymes have previously been identified and characterized in bacteria and the alga Chlamydomonas reinhardtii. We now report that many fungal genomes, including those of plant and animal pathogens, encode the enzymatic machinery for DGTS biosynthesis, and that fungi synthesize DGTS during P limitation. This finding demonstrates that replacement of phospholipids by nonphosphorous lipids is a strategy used in divergent eukaryotic lineages for the conservation of P under P-limiting conditions. Mutants of Neurospora crassa were used to show that DGTS synthase encoded by the BTA1 gene is solely responsible for DGTS biosynthesis and is under the control of the fungal phosphorus deprivation regulon, mediated by the NUC-1/Pho4p transcription factor. Furthermore, we describe the rational reengineering of lipid metabolism in the yeast Saccharomyces cerevisiae, such that PtdCho is completely replaced by DGTS, and demonstrate that essential processes of membrane biogenesis and organelle assembly are functional and support growth in the engineered strain.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

