Repair of segmental bone defect using Totally Vitalized tissue engineered bone graft by a combined perfusion seeding and culture system
- PMID: 24728277
- PMCID: PMC3984127
- DOI: 10.1371/journal.pone.0094276
Repair of segmental bone defect using Totally Vitalized tissue engineered bone graft by a combined perfusion seeding and culture system
Abstract
Background: The basic strategy to construct tissue engineered bone graft (TEBG) is to combine osteoblastic cells with three dimensional (3D) scaffold. Based on this strategy, we proposed the "Totally Vitalized TEBG" (TV-TEBG) which was characterized by abundant and homogenously distributed cells with enhanced cell proliferation and differentiation and further investigated its biological performance in repairing segmental bone defect.
Methods: In this study, we constructed the TV-TEBG with the combination of customized flow perfusion seeding/culture system and β-tricalcium phosphate (β-TCP) scaffold fabricated by Rapid Prototyping (RP) technique. We systemically compared three kinds of TEBG constructed by perfusion seeding and perfusion culture (PSPC) method, static seeding and perfusion culture (SSPC) method, and static seeding and static culture (SSSC) method for their in vitro performance and bone defect healing efficacy with a rabbit model.
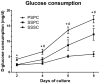
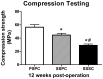
Results: Our study has demonstrated that TEBG constructed by PSPC method exhibited better biological properties with higher daily D-glucose consumption, increased cell proliferation and differentiation, and better cell distribution, indicating the successful construction of TV-TEBG. After implanted into rabbit radius defects for 12 weeks, PSPC group exerted higher X-ray score close to autograft, much greater mechanical property evidenced by the biomechanical testing and significantly higher new bone formation as shown by histological analysis compared with the other two groups, and eventually obtained favorable healing efficacy of the segmental bone defect that was the closest to autograft transplantation.
Conclusion: This study demonstrated the feasibility of TV-TEBG construction with combination of perfusion seeding, perfusion culture and RP technique which exerted excellent biological properties. The application of TV-TEBG may become a preferred candidate for segmental bone defect repair in orthopedic and maxillofacial fields.
Conflict of interest statement
Figures








References
-
- Kruyt MC, de Bruijn JD, Wilson CE, Oner FC, van Blitterswijk CA, et al. (2003) Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng 9: 327–336. - PubMed
-
- Zhang ZY, Teoh SH, Hui JH, Fisk NM, Choolani M, et al. (2012) The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials 33: 2656–2672. - PubMed
-
- Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT (2008) Tissue engineering of bone: material and matrix considerations. The Journal of bone and joint surgery American volume 90 Suppl 136–42. - PubMed
-
- Ge Z, Baguenard S, Lim LY, Wee A, Khor E (2004) Hydroxyapatite-chitin materials as potential tissue engineered bone substitutes. Biomaterials 25: 1049–1058. - PubMed
-
- Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, et al. (1998) Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog 14: 193–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

