Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration
- PMID: 24728329
- PMCID: PMC4112421
- DOI: 10.1136/annrheumdis-2014-205228
Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration
Abstract
Objective: To compare the relative bioavailability, safety and tolerability of oral methotrexate (MTX) and subcutaneous (SC) MTX administered via an auto-injector (MTXAI) in patients with rheumatoid arthritis (RA).
Methods: In this randomised, multicenter, open-label, three-way crossover study, patients ≥18 years with adult RA undergoing treatment with MTX for ≥3 months were assigned to receive MTX 10, 15, 20 and 25 mg weekly in a random sequence of three treatments: oral, SC into the abdomen and SC into the thigh. For 24 h after administration of each treatment, blood samples were collected for pharmacokinetic analysis and injection sites were assessed.
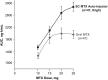
Results: Forty-seven patients completed the study. Systemic exposure of oral MTX plateaued at doses ≥15 mg/week. In contrast, SC MTX demonstrated a linear increase in systemic exposure that was greater than oral MTX at each dose. No unexpected AEs were noted for either formulation.
Conclusions: Unlike oral MTX, the systemic exposure of SC MTX did not plateau over the doses studied, particularly at doses ≥15 mg/week. In this study, higher systemic MTX exposure was not associated with increases in AEs. Patients with an inadequate clinical response to oral MTX may benefit from higher drug exposure by switching to SC MTX.
Trial registration number: NCT01618968.
Keywords: Methotrexate; Pharmacokinetics; Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Comment in
-
Is there a direct relationship between serum level of methotrexate and clinical efficacy and tolerability?Ann Rheum Dis. 2014 Sep;73(9):e54. doi: 10.1136/annrheumdis-2014-205901. Epub 2014 Jun 3. Ann Rheum Dis. 2014. PMID: 24894190 No abstract available.
-
Response to: 'is there a direct relationship between serum level of methotrexate and clinical efficacy and tolerability?' by Fleishman and Yazici.Ann Rheum Dis. 2014 Sep;73(9):e55. doi: 10.1136/annrheumdis-2014-205938. Epub 2014 Jun 24. Ann Rheum Dis. 2014. PMID: 24962874 No abstract available.
References
-
- Buxton I. Pharmacokinetics and Pharmacodynamics. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11 ed. USA: The McGraw-Hill Companies, Inc., 2006:1–39
-
- Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol 1997;36:86–90 - PubMed
-
- Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol 2004;31:645–8 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical