Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen
- PMID: 24729031
- PMCID: PMC4145421
- DOI: 10.1136/bjophthalmol-2013-304556
Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen
Abstract
Aims: The purpose of this study was to clinically validate an individually planned treatment regimen for neovascular age-related macular degeneration (nAMD), termed, observe and plan. This regimen was based on the predictability of an individual's need for retreatment and aimed to reduce the clinical burden, while obtaining good functional results.
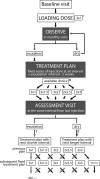
Methods: This was a prospective case series that included 104 patients (115 eyes) with treatment-naive nAMD. Following three loading doses of ranibizumab, monthly observation visits allowed the disease recurrence interval to be determined. The recurrence interval was reduced by 2 weeks to give the retreatment interval for the next three injections. Periodical control visits (at least every 6 months) allowed the effectiveness of the treatment to be assessed and individual intervals adjusted.
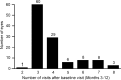
Results: Mean visual acuity (VA) improved by 8.7 and 9.8 letters in months 3 and 12, respectively. The mean number of injections during the 12-month study was 7.8, while the mean number of ophthalmic examinations between months 3 and 12 was 3.97. The mean treatment interval after the loading doses was 1.97 months.
Conclusions: The observe-and-plan regimen significantly improved VA. This was obtained with fewer clinic visits compared with other regimens, which could ease the burden of nAMD treatment.
Trial registration number: Commission cantonale (VD) d'éthique de la recherché Clinique, Université de Lausanne, Protocole 351/11.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859–70 - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31 - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44 - PubMed
-
- Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009;116:57–65 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
