Intestinal tumor suppression in ApcMin/+ mice by prostaglandin D2 receptor PTGDR
- PMID: 24729479
- PMCID: PMC4303173
- DOI: 10.1002/cam4.251
Intestinal tumor suppression in ApcMin/+ mice by prostaglandin D2 receptor PTGDR
Abstract
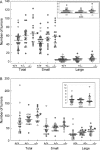
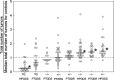
Our earlier work showed that knockout of hematopoietic prostaglandin D synthase (HPGDS, an enzyme that produces prostaglandin D2) caused more adenomas in Apc(Min/+) mice. Conversely, highly expressed transgenic HPGDS allowed fewer tumors. Prostaglandin D2 (PGD2) binds to the prostaglandin D2 receptor known as PTGDR (or DP1). PGD2 metabolites bind to peroxisome proliferator-activated receptor γ (PPARG). We hypothesized that Ptgdr or Pparg knockouts may raise numbers of tumors, if these receptors take part in tumor suppression by PGD2. To assess, we produced Apc(Min/+) mice with and without Ptgdr knockouts (147 mice). In separate experiments, we produced Apc(Min/+) mice expressing transgenic lipocalin-type prostaglandin D synthase (PTGDS), with and without heterozygous Pparg knockouts (104 mice). Homozygous Ptgdr knockouts raised total numbers of tumors by 30-40% at 6 and 14 weeks. Colon tumors were not affected. Heterozygous Pparg knockouts alone did not affect tumor numbers in Apc(Min/+) mice. As mentioned above, our Pparg knockout assessment also included mice with highly expressed PTGDS transgenes. Apc(Min/+) mice with transgenic PTGDS had fewer large adenomas (63% of control) and lower levels of v-myc avian myelocytomatosis viral oncogene homolog (MYC) mRNA in the colon. Heterozygous Pparg knockouts appeared to blunt the tumor-suppressing effect of transgenic PTGDS. However, tumor suppression by PGD2 was more clearly mediated by receptor PTGDR in our experiments. The suppression mechanism did not appear to involve changes in microvessel density or slower proliferation of tumor cells. The data support a role for PGD2 signals acting through PTGDR in suppression of intestinal tumors.
Keywords: Adenomatous polyposis coli; PPAR gamma; gastrointestinal neoplasms; prostaglandin D2 receptor; prostaglandin D2 synthases.
© 2014 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures


References
-
- Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. - PubMed
-
- Park JM, Kanaoka Y, Eguchi N, Aritake K, Grujic S, Materi AM, et al. Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. Cancer Res. 2007;67:881–889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

