Role of autophagy and apoptosis in wound tissue of deep second-degree burn in rats
- PMID: 24730400
- PMCID: PMC4114170
- DOI: 10.1111/acem.12352
Role of autophagy and apoptosis in wound tissue of deep second-degree burn in rats
Abstract
Objectives: The pathogenesis of burn wound progression is poorly understood. Contributing factors include continuous loss of blood perfusion, excessive inflammation, and elevated apoptosis levels in wound tissue. Macroautophagy (here referred to simply as "autophagy") is associated with many chronic diseases. The authors hypothesized that autophagy is involved in burn wound progression in a rat model of deep second-degree burn.
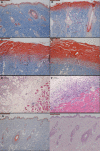
Methods: Deep second-degree burns were modeled using a brass rod heated to 100°C applied for 6 seconds to the back skin of Wistar rats. Full-thickness biopsies were obtained from burned and nonburned controls at several times postburn. Western blotting and immunohistochemical (IHC) staining determined expression of the autophagy markers Light Chain 3 (LC3) and beclin-1. Apoptosis was determined by terminal-deoxynucleoitidyl transferase mediated nick end labeling (TUNEL) assay and laser Doppler flowmetry (LDF)-measured tissue perfusion. Myeloperoxidase (MPO) activity assay measured inflammation. Hematoxylin and eosin (H&E) and Masson's trichrome staining-determined pathology and wound depth.
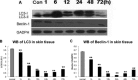
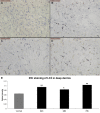
Results: The LC3 and beclin-1 protein level in burn wounds decreased to one-fourth of normal levels (p<0.01) over 24 hours and then began to increase but still did not reach their normal level. TUNEL-positive cells in burn wounds were 3.7-fold (p<0.01) elevated over 48 hours and then decreased slightly, yet still remained higher than in normal skin. The burn wound progressed in depth over 72 hours. In addition, significant decrease in LDF values and upregulation of MPO activity were observed. Enhanced LC3-positive cells were observed in the deep dermal layer of burn wounds as shown by IHC staining.
Conclusions: A reduction in autophagy and blood flow and an increase in apoptosis and inflammation were observed in burn wounds early during the course of burn injury progression. This suggests that autophagy, complemented by apoptosis, play important roles in burn progression. Enhanced autophagy in the deep dermis may be a prosurvival mechanism against ischemia and inflammation after burn injury.
Objetivos: La patogénesis de la progresión en la lesión por quemadura es poco conocida. Los factores contribuyentes incluyen la pérdida continua de perfusión sanguínea, la excesiva inflamación y los marcadores de apoptosis elevados en el tejido lesionado. La macroautofagia (aquí referida simplemente como “autofagia”) se asocia con muchas enfermedades crónicas. Se generó la hipótesis que la autofagia está implicada en la progresión de la lesión por quemadura en un modelo de quemadura de segundo grado en ratas.
Metodología:
Las quemaduras de segundo grado se simularon usando un varilla de metal calentada hasta 100°C y se aplicó durante 6 segundos en la piel del dorso de las ratas Wistar. Se obtuvieron biopsias de todo el grosor de los quemados y de los controles no quemados en diversas ocasiones tras la quemadura. Se determinó la expresión de marcadores de autofagia Light Chain 3 (
Resultados:
El valor de proteínas Beclin‐1 y
Conclusiones: Se observaron una reducción de la autofagia y del flujo sanguíneo y un incremento en la apoptosis y la inflamación en las heridas por quemadura al inicio del curso de la progresión de la lesión. Esto indica que la autofagia, junto con la apoptosis, juegan papeles importantes en la progresión de la quemadura. El incremento de la autofagia en la dermis profunda puede ser un mecanismo a favor de la supervivencia y que actúa en contra de la isquemia y la inflamación tras la lesión por quemadura.
© 2014 The Authors. Academic Emergency Medicine published by Wiley Periodicals, Inc. on behalf of Society for Academic Emergency Medicine (SAEM).
Conflict of interest statement
The authors have no relevant financial information or potential conflicts of interest to disclose.
Figures

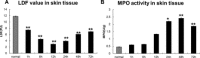
Similar articles
-
Rapamycin reduces burn wound progression by enhancing autophagy in deep second-degree burn in rats.Wound Repair Regen. 2013 Nov-Dec;21(6):852-9. doi: 10.1111/wrr.12090. Epub 2013 Aug 23. Wound Repair Regen. 2013. PMID: 23980869
-
The roles of autophagy and apoptosis in burn wound progression in rats.Burns. 2013 Dec;39(8):1551-6. doi: 10.1016/j.burns.2013.04.018. Epub 2013 Jun 14. Burns. 2013. PMID: 23751274
-
3,4-Methylenedioxy-β-Nitrostyrene Ameliorates Experimental Burn Wound Progression by Inhibiting the NLRP3 Inflammasome Activation.Plast Reconstr Surg. 2016 Mar;137(3):566e-575e. doi: 10.1097/01.prs.0000479972.06934.83. Plast Reconstr Surg. 2016. PMID: 26910701
-
Role of autophagy and its molecular mechanisms in mice intestinal tract after severe burn.J Trauma Acute Care Surg. 2017 Oct;83(4):716-724. doi: 10.1097/TA.0000000000001624. J Trauma Acute Care Surg. 2017. PMID: 28930963
-
[Research advances on the prevention and treatment strategies of burn wound progressive deepening].Zhonghua Shao Shang Za Zhi. 2021 Dec 20;37(12):1199-1204. doi: 10.3760/cma.j.cn501120-20200828-00396. Zhonghua Shao Shang Za Zhi. 2021. PMID: 34937157 Free PMC article. Review. Chinese.
Cited by
-
Comprehensive perception of burn conversion: a literature review.Ann Burns Fire Disasters. 2020 Jun 30;33(2):89-96. Ann Burns Fire Disasters. 2020. PMID: 32913427 Free PMC article.
-
Far-infrared promotes burn wound healing by suppressing NLRP3 inflammasome caused by enhanced autophagy.J Mol Med (Berl). 2016 Jul;94(7):809-19. doi: 10.1007/s00109-016-1389-0. Epub 2016 Feb 11. J Mol Med (Berl). 2016. PMID: 26864306
-
A Critical Update of the Assessment and Acute Management of Patients with Severe Burns.Adv Wound Care (New Rochelle). 2019 Dec 1;8(12):607-633. doi: 10.1089/wound.2019.0963. Epub 2019 Nov 6. Adv Wound Care (New Rochelle). 2019. PMID: 31827977 Free PMC article. Review.
-
Simulated aeromedical evacuation exacerbates burn induced lung injury: targeting mitochondrial DNA for reversal.Mil Med Res. 2021 May 13;8(1):30. doi: 10.1186/s40779-021-00320-9. Mil Med Res. 2021. PMID: 33985568 Free PMC article.
-
Silver Decorated Mesoporous Carbons for the Treatment of Acute and Chronic Wounds, in a Tissue Regeneration Context.Int J Nanomedicine. 2019 Dec 31;14:10147-10164. doi: 10.2147/IJN.S234393. eCollection 2019. Int J Nanomedicine. 2019. PMID: 32021158 Free PMC article.
References
-
- Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plastic Surg 2007;59:109–15 - PubMed
-
- Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–73 - PubMed
-
- Kao CC, Garner WL. Acute burns. Plastic Reconstr Surg 2000;101:2482–93 - PubMed
-
- Gravante G, Palmieri MB, Esposito G, et al. Apoptotic death in deep partial thickness burns vs. normal skin of burned patients. J Surg Res 2007;141:141–5 - PubMed
-
- Singer AJ, McClain SA, Taira BR, Guerriero JL, Zong W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med 2008;15:549–54 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

