The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer
- PMID: 24730679
- PMCID: PMC4307161
- DOI: 10.1089/ars.2014.5933
The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer
Abstract
Significance: Cancer represents a major socioeconomic problem; there is a significant need for novel therapeutic approaches targeting tumor-specific pathways.
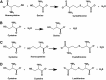
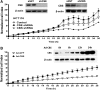
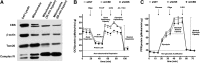
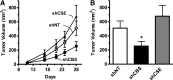
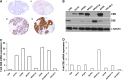
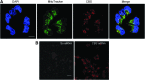
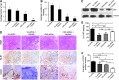
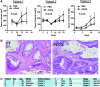
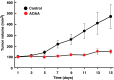
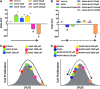
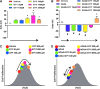
Recent advances: In colorectal and ovarian cancers, an increase in the intratumor production of hydrogen sulfide (H2S) from cystathionine β-synthase (CBS) plays an important role in promoting the cellular bioenergetics, proliferation, and migration of cancer cells. It also stimulates peritumor angiogenesis inhibition or genetic silencing of CBS exerts antitumor effects both in vitro and in vivo, and potentiates the antitumor efficacy of anticancer therapeutics.
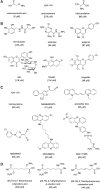
Critical issues: Recently published studies are reviewed, implicating CBS overexpression and H2S overproduction in tumor cells as a tumor-growth promoting "bioenergetic fuel" and "survival factor," followed by an overview of the experimental evidence demonstrating the anticancer effect of CBS inhibition. Next, the current state of the art of pharmacological CBS inhibitors is reviewed, with special reference to the complex pharmacological actions of aminooxyacetic acid. Finally, new experimental evidence is presented to reconcile a controversy in the literature regarding the effects of H2S donor on cancer cell proliferation and survival.
Future directions: From a basic science standpoint, future directions in the field include the delineation of the molecular mechanism of CBS up-regulation of cancer cells and the delineation of the interactions of H2S with other intracellular pathways of cancer cell metabolism and proliferation. From the translational science standpoint, future directions include the translation of the recently emerging roles of H2S in cancer into human diagnostic and therapeutic approaches.
Figures








References
-
- Abraham JM. and Cho L. The homocysteine hypothesis: still relevant to the prevention and treatment of cardiovascular disease? Cleve Clin J Med 77: 911–918, 2010 - PubMed
-
- Akahoshi N, Kobayashi C, Ishizaki Y, Izumi T, Himi T, Suematsu M, and Ishii I. Genetic background conversion ameliorates semi-lethality and permits behavioral analyses in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. Hum Mol Genet 17: 1994–2005, 2008 - PubMed
-
- Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, Tutino M, Dragonieri S, Memeo V, and de Gennaro G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 100: 144–150, 2013 - PubMed
-
- Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, and Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169: 922–932, 2013 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

