Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome
- PMID: 24731444
- PMCID: PMC4044720
- DOI: 10.1016/j.ajpath.2014.02.027
Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome
Abstract
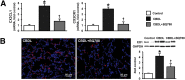
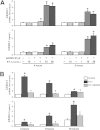
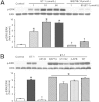
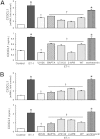
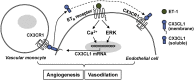
Hepatic production and release of endothelin-1 (ET-1) binding to endothelin B (ETB) receptors, overexpressed in the lung microvasculature, is associated with accumulation of pro-angiogenic monocytes and vascular remodeling in experimental hepatopulmonary syndrome (HPS) after common bile duct ligation (CBDL). We have recently found that lung vascular monocyte adhesion and angiogenesis in HPS involve interaction of endothelial C-X3-C motif ligand 1 (CX3CL1) with monocyte CX3C chemokine receptor 1 (CX3CR1), although whether ET-1/ETB receptor activation influences these events is unknown. Our aim was to define if ET-1/ETB receptor activation modulates CX3CL1/CX3CR1 signaling and lung angiogenesis in experimental HPS. A selective ETB receptor antagonist, BQ788, was given for 2 weeks to 1-week CBDL rats. ET-1 (±BQ788) was given to cultured rat pulmonary microvascular endothelial cells overexpressing ETB receptors. BQ788 treatment significantly decreased lung angiogenesis, monocyte accumulation, and CX3CL1 levels after CBDL. ET-1 treatment significantly induced CX3CL1 production in lung microvascular endothelial cells, which was blocked by inhibitors of Ca(2+) and mitogen-activated protein kinase (MEK)/ERK pathways. ET-1-induced ERK activation was Ca(2+) independent. ET-1 administration also increased endothelial tube formation in vitro, which was inhibited by BQ788 or by blocking Ca(2+) and MEK/ERK activation. CX3CR1 neutralizing antibody partially inhibited ET-1 effects on tube formation. These findings identify a novel mechanistic interaction between the ET-1/ETB receptor axis and CX3CL1/CX3CR1 in mediating pulmonary angiogenesis and vascular monocyte accumulation in experimental HPS.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome.J Hepatol. 2012 Oct;57(4):752-8. doi: 10.1016/j.jhep.2012.05.014. Epub 2012 May 29. J Hepatol. 2012. PMID: 22659346 Free PMC article.
-
The role of receptor tyrosine kinase activation in cholangiocytes and pulmonary vascular endothelium in experimental hepatopulmonary syndrome.Am J Physiol Gastrointest Liver Physiol. 2014 Jan 1;306(1):G72-80. doi: 10.1152/ajpgi.00178.2013. Epub 2013 Nov 7. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24200956 Free PMC article.
-
Attenuation of experimental hepatopulmonary syndrome in endothelin B receptor-deficient rats.Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G704-8. doi: 10.1152/ajpgi.90627.2008. Epub 2009 Feb 5. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19196949 Free PMC article.
-
The endothelin system in pulmonary arterial hypertension.Cardiovasc Res. 2004 Feb 1;61(2):227-37. doi: 10.1016/j.cardiores.2003.11.026. Cardiovasc Res. 2004. PMID: 14736539 Review.
-
CX3CL1 (Fractalkine)-CX3CR1 Axis in Inflammation-Induced Angiogenesis and Tumorigenesis.Int J Mol Sci. 2024 Apr 25;25(9):4679. doi: 10.3390/ijms25094679. Int J Mol Sci. 2024. PMID: 38731899 Free PMC article. Review.
Cited by
-
Hepatopulmonary Syndrome and Liver Transplantation: A Recent Review of the Literature.J Clin Transl Hepatol. 2016 Mar 28;4(1):47-53. doi: 10.14218/JCTH.2015.00044. Epub 2016 Mar 15. J Clin Transl Hepatol. 2016. PMID: 27047772 Free PMC article. Review.
-
Role of MicroRNA-103a Targeting ADAM10 in Abdominal Aortic Aneurysm.Biomed Res Int. 2017;2017:9645874. doi: 10.1155/2017/9645874. Epub 2017 Mar 5. Biomed Res Int. 2017. PMID: 28357407 Free PMC article.
-
Novel immunotargets in multiple myeloma: biological relevance and therapeutic potential.Biomark Res. 2025 Jul 1;13(1):92. doi: 10.1186/s40364-025-00799-7. Biomark Res. 2025. PMID: 40597436 Free PMC article. Review.
-
Requirement of miR-9-dependent regulation of Myocd in PASMCs phenotypic modulation and proliferation induced by hepatopulmonary syndrome rat serum.J Cell Mol Med. 2015 Oct;19(10):2453-61. doi: 10.1111/jcmm.12631. Epub 2015 Jul 6. J Cell Mol Med. 2015. PMID: 26147104 Free PMC article.
-
Plasma Reticulocalbin 3 (RCN3) is a Novel Biomarker for the Early Diagnosis of Hepatopulmonary Syndrome in Cirrhotic Patients.Lung. 2025 Mar 20;203(1):50. doi: 10.1007/s00408-025-00807-5. Lung. 2025. PMID: 40111510
References
-
- Cahill P.A., Redmond E.M., Sitzmann J.V. Endothelial dysfunction in cirrhosis and portal hypertension. Pharmacol Ther. 2001;89:273–293. - PubMed
-
- Lee J.S., Semela D., Iredale J., Shah V.H. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. - PubMed
-
- Rodriguez-Roisin R., Krowka M.J. Hepatopulmonary syndrome: a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. - PubMed
-
- Rodriguez-Roisin R., Krowka M.J., Herve P., Fallon M.B., ERS Task Force Pulmonary-Hepatic Vascular Disorders Scientific Committee ERS Task Force PHD Scientific Committee Pulmonary-hepatic vascular disorders (PHD) Eur Respir J. 2004;24:861–880. - PubMed
-
- Schenk P., Schoniger-Hekele M., Fuhrmann V., Madl C., Silberhumer G., Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

