A cost-utility analysis of drug treatments in patients with HBeAg-positive chronic hepatitis B in Thailand
- PMID: 24731689
- PMCID: PMC3996169
- DOI: 10.1186/1472-6963-14-170
A cost-utility analysis of drug treatments in patients with HBeAg-positive chronic hepatitis B in Thailand
Abstract
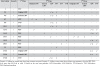
Background: Only lamivudine has been included for patients with chronic hepatitis B (CHB) in the National List of Essential Drugs (NLED), a pharmaceutical reimbursement list in Thailand. There have also been no economic evaluation studies of CHB drug treatments conducted in Thailand yet. In order to fill this gap in policy research, the objective of this study was to compare the cost-utility of each drug therapy (Figure 1) with palliative care in patients with HBeAg-positive CHB.
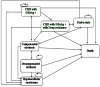
Methods: A cost-utility analysis using an economic evaluation model was performed to compare each drug treatment for HBeAg-positive CHB patients. A Markov model was used to estimate the relevant costs and health outcomes during a lifetime horizon based on a societal perspective. Direct medical costs, direct non-medical costs, and indirect costs were included, and health outcomes were denoted in life years (LYs) and quality-adjusted life years (QALYs). The results were presented as an incremental cost effectiveness ratio (ICER) in Thai baht (THB) per LY or QALY gained. One-way sensitivity and probabilistic sensitivity analyses were applied to investigate the effects of model parameter uncertainties.
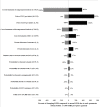
Results: The ICER values of providing generic lamivudine with the addition of tenofovir when drug resistance occurred, generic lamivudine with the addition of tenofovir based on the road map guideline, and tenofovir monotherapy were -14,000 (USD -467), -8,000 (USD -267) , and -5,000 (USD -167) THB per QALY gained, respectively. However, when taking into account all parameter uncertainties in the model, providing generic lamivudine with the addition of tenofovir when drug resistance occurred (78% and 75%) and tenofovir monotherapy (18% and 24%) would yield higher probabilities of being cost-effective at the societal willingness to pay thresholds of 100,000 (USD 3,333) and 300,000 (USD 10,000) THB per QALY gained in Thailand, respectively.
Conclusions: Based on the policy recommendations from this study, the Thai government decided to include tenofovir into the NLED in addition to generic lamivudine which is already on the list. Moreover, the results have shown that the preferred treatment regimen involves using generic lamivudine as the first-line drug with tenofovir added if drug resistance occurs in HBeAg-positive CHB patients.
Figures

Similar articles
-
Cost effectiveness of tenofovir disoproxil fumarate for the treatment of chronic hepatitis B from a Canadian public payer perspective.Pharmacoeconomics. 2011 Dec;29(12):1075-91. doi: 10.2165/11589260-000000000-00000. Pharmacoeconomics. 2011. PMID: 22077579
-
Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B infection.Health Technol Assess. 2010 May;14 Suppl 1:23-9. doi: 10.3310/hta14Suppl1/04. Health Technol Assess. 2010. PMID: 20507800 Review.
-
Cost-effectiveness analysis of different rescue therapies in patients with lamivudine-resistant chronic hepatitis B in China.BMC Health Serv Res. 2012 Nov 8;12:385. doi: 10.1186/1472-6963-12-385. BMC Health Serv Res. 2012. PMID: 23137013 Free PMC article.
-
Cost effectiveness of entecavir versus lamivudine with adefovir salvage in HBeAg-positive chronic hepatitis B.Pharmacoeconomics. 2007;25(11):963-77. doi: 10.2165/00019053-200725110-00006. Pharmacoeconomics. 2007. PMID: 17960954 Clinical Trial.
-
Economic evaluation of treatments for chronic hepatitis B.Braz J Infect Dis. 2013 Jul-Aug;17(4):418-26. doi: 10.1016/j.bjid.2012.12.005. Epub 2013 Jul 10. Braz J Infect Dis. 2013. PMID: 23849851 Free PMC article.
Cited by
-
Development of a Health Screening Package Under the Universal Health Coverage: The Role of Health Technology Assessment.Health Econ. 2016 Feb;25 Suppl 1(Suppl Suppl 1):162-78. doi: 10.1002/hec.3301. Epub 2016 Jan 15. Health Econ. 2016. PMID: 26774008 Free PMC article.
-
A Cost-Utility and Cost-Effectiveness Analysis of Different Oral Antiviral Medications in Patients With HBeAg-Negative Chronic Hepatitis B in Iran: An Economic Microsimulation Decision Model.Hepat Mon. 2016 Aug 14;16(9):e37435. doi: 10.5812/hepatmon.37435. eCollection 2016 Sep. Hepat Mon. 2016. PMID: 27822262 Free PMC article.
-
Are Published Health Economic Models for Chronic Hepatitis B Appropriately Capturing the Benefits of HBsAg Loss? A Systematic Literature Review.Pharmacoecon Open. 2020 Sep;4(3):403-418. doi: 10.1007/s41669-019-00175-w. Pharmacoecon Open. 2020. PMID: 31428938 Free PMC article. Review.
-
Cost-Effectiveness of Peg-Interferon, Interferon and Oral Nucleoside Analogues in the Treatment of Chronic Hepatitis B and D Infections in China.Clin Drug Investig. 2016 Aug;36(8):637-48. doi: 10.1007/s40261-016-0409-8. Clin Drug Investig. 2016. PMID: 27166628
-
A Cost-Utility Analysis of Different Antiviral Medicine Regimens in Patients With Chronic Hepatitis C Virus Genotype 1 Infection.Iran Red Crescent Med J. 2016 Oct 2;18(11):e37094. doi: 10.5812/ircmj.37094. eCollection 2016 Nov. Iran Red Crescent Med J. 2016. PMID: 28203449 Free PMC article.
References
-
- Hepatitis B. [ http://www.who.int/immunization/topics/hepatitis_b/en/]
-
- van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Hüppe D, Stein K, Trojan J, Sarrazin C, Böcher WO, Spengler U, Wasmuth HE, Reinders JG, Möller B, Rhode P, Feucht HH, Wiedenmann B, Berg T. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;14(1):73–80. doi: 10.1002/hep.23246. - DOI - PubMed
-
- Chen W, Hou JL. Pharmacoeconomic evaluation of telbivudine vs. lamivudine in treating the patients with HBeAg-positive and negative chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2009;14(8):569–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

