Spores of Clostridium engineered for clinical efficacy and safety cause regression and cure of tumors in vivo
- PMID: 24732092
- PMCID: PMC4039107
- DOI: 10.18632/oncotarget.1761
Spores of Clostridium engineered for clinical efficacy and safety cause regression and cure of tumors in vivo
Abstract
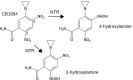
Spores of some species of the strictly anaerobic bacteria Clostridium naturally target and partially lyse the hypoxic cores of tumors, which tend to be refractory to conventional therapies. The anti-tumor effect can be augmented by engineering strains to convert a non-toxic prodrug into a cytotoxic drug specifically at the tumor site by expressing a prodrug-converting enzyme (PCE). Safe doses of the favored prodrug CB1954 lead to peak concentrations of 6.3 µM in patient sera, but at these concentration(s) known nitroreductase (NTR) PCEs for this prodrug show low activity. Furthermore, efficacious and safe Clostridium strains that stably express a PCE have not been reported. Here we identify a novel nitroreductase from Neisseria meningitidis, NmeNTR, which is able to activate CB1954 at clinically-achievable serum concentrations. An NmeNTR expression cassette, which does not contain an antibiotic resistance marker, was stably localized to the chromosome of Clostridium sporogenes using a new integration method, and the strain was disabled for safety and containment by making it a uracil auxotroph. The efficacy of Clostridium-Directed Enzyme Prodrug Therapy (CDEPT) using this system was demonstrated in a mouse xenograft model of human colon carcinoma. Substantial tumor suppression was achieved, and several animals were cured. These encouraging data suggest that the novel enzyme and strain engineering approach represent a promising platform for the clinical development of CDEPT.
Figures


Similar articles
-
Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo.Br J Cancer. 2006 Nov 6;95(9):1212-9. doi: 10.1038/sj.bjc.6603367. Epub 2006 Oct 3. Br J Cancer. 2006. PMID: 17024128 Free PMC article.
-
Chemotherapeutic tumour targeting using clostridial spores.FEMS Microbiol Rev. 1995 Oct;17(3):357-64. doi: 10.1111/j.1574-6976.1995.tb00219.x. FEMS Microbiol Rev. 1995. PMID: 7576773 Review.
-
Discovery and evaluation of Escherichia coli nitroreductases that activate the anti-cancer prodrug CB1954.Biochem Pharmacol. 2010 Mar 1;79(5):678-87. doi: 10.1016/j.bcp.2009.10.008. Epub 2009 Oct 21. Biochem Pharmacol. 2010. PMID: 19852945
-
The bystander effect of the nitroreductase/CB1954 enzyme/prodrug system is due to a cell-permeable metabolite.Hum Gene Ther. 1997 Apr 10;8(6):709-17. doi: 10.1089/hum.1997.8.6-709. Hum Gene Ther. 1997. PMID: 9113510
-
Virus-directed enzyme prodrug therapy using CB1954.Anticancer Drug Des. 1999 Dec;14(6):461-72. Anticancer Drug Des. 1999. PMID: 10834268 Review.
Cited by
-
Heterologous Gene Regulation in Clostridia: Rationally Designed Gene Regulation for Industrial and Medical Applications.ACS Synth Biol. 2022 Nov 18;11(11):3817-3828. doi: 10.1021/acssynbio.2c00401. Epub 2022 Oct 20. ACS Synth Biol. 2022. PMID: 36265075 Free PMC article.
-
Genome sequence of Clostridium sporogenes DSM 795(T), an amino acid-degrading, nontoxic surrogate of neurotoxin-producing Clostridium botulinum.Stand Genomic Sci. 2015 Jul 21;10:40. doi: 10.1186/s40793-015-0016-y. eCollection 2015. Stand Genomic Sci. 2015. PMID: 26221421 Free PMC article.
-
Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: Set the bugs to work?Biotechnol Adv. 2018 Jul-Aug;36(4):968-985. doi: 10.1016/j.biotechadv.2018.02.016. Epub 2018 Feb 28. Biotechnol Adv. 2018. PMID: 29499341 Free PMC article. Review.
-
A roadmap for gene system development in Clostridium.Anaerobe. 2016 Oct;41:104-112. doi: 10.1016/j.anaerobe.2016.05.011. Epub 2016 May 24. Anaerobe. 2016. PMID: 27234263 Free PMC article. Review.
-
Clostridium Bacteria: Harnessing Tumour Necrosis for Targeted Gene Delivery.Mol Diagn Ther. 2024 Mar;28(2):141-151. doi: 10.1007/s40291-024-00695-0. Epub 2024 Feb 2. Mol Diagn Ther. 2024. PMID: 38302842 Free PMC article.
References
-
- NIH. Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2012;13:3–13. - PubMed
-
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. - PMC - PubMed
-
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R, Pelusio A, Le Boeuf F, Burns J, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. - PubMed
-
- Mengesha A, Dubois L, Chiu RK, Paesmans K, Wouters BG, Lambin P, Theys J. Potential and limitations of bacterial-mediated cancer therapy. Front Biosci. 2007;12:3880–3891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

