Compartments within a compartment: what C. elegans can tell us about ciliary subdomain composition, biogenesis, function, and disease
- PMID: 24732235
- PMCID: PMC4049889
- DOI: 10.4161/org.28830
Compartments within a compartment: what C. elegans can tell us about ciliary subdomain composition, biogenesis, function, and disease
Abstract
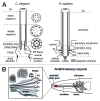
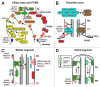
The primary cilium has emerged as a hotbed of sensory and developmental signaling, serving as a privileged domain to concentrate the functions of a wide number of channels, receptors and downstream signal transducers. This realization has provided important insight into the pathophysiological mechanisms underlying the ciliopathies, an ever expanding spectrum of multi-symptomatic disorders affecting the development and maintenance of multiple tissues and organs. One emerging research focus is the subcompartmentalised nature of the organelle, consisting of discrete structural and functional subdomains such as the periciliary membrane/basal body compartment, the transition zone, the Inv compartment and the distal segment/ciliary tip region. Numerous ciliopathy, transport-related and signaling molecules localize at these compartments, indicating specific roles at these subciliary sites. Here, by focusing predominantly on research from the genetically tractable nematode C. elegans, we review ciliary subcompartments in terms of their structure, function, composition, biogenesis and relationship to human disease.
Keywords: basal body; cilia; ciliary tip; ciliopathy; diffusion barrier; distal segment; intraflagellar transport; inversin compartment; periciliary membrane; transition zone.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
