Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis
- PMID: 24732289
- PMCID: PMC3986205
- DOI: 10.1371/journal.pone.0094462
Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis
Abstract
Rationale: Sutezolid (PNU-100480) is a linezolid analog with superior bactericidal activity against Mycobacterium tuberculosis in the hollow fiber, whole blood and mouse models. Like linezolid, it is unaffected by mutations conferring resistance to standard TB drugs. This study of sutezolid is its first in tuberculosis patients.
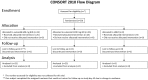
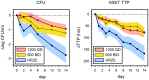
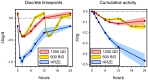
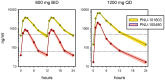
Methods: Sputum smear positive tuberculosis patients were randomly assigned to sutezolid 600 mg BID (N = 25) or 1200 mg QD (N = 25), or standard 4-drug therapy (N = 9) for the first 14 days of treatment. Effects on mycobacterial burden in sputum (early bactericidal activity or EBA) were monitored as colony counts on agar and time to positivity in automated liquid culture. Bactericidal activity was also measured in ex vivo whole blood cultures (whole blood bactericidal activity or WBA) inoculated with M. tuberculosis H37Rv.
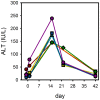
Results: All patients completed assigned treatments and began subsequent standard TB treatment according to protocol. The 90% confidence intervals (CI) for bactericidal activity in sputum over the 14 day interval excluded zero for all treatments and both monitoring methods, as did those for cumulative WBA. There were no treatment-related serious adverse events, premature discontinuations, or dose reductions due to laboratory abnormalities. There was no effect on the QT interval. Seven sutezolid-treated patients (14%) had transient, asymptomatic ALT elevations to 173±34 U/L on day 14 that subsequently normalized promptly; none met Hy's criteria for serious liver injury.
Conclusions: The mycobactericidal activity of sutezolid 600 mg BID or 1200 mg QD was readily detected in sputum and blood. Both schedules were generally safe and well tolerated. Further studies of sutezolid in tuberculosis treatment are warranted.
Trial registration: ClinicalTrials.gov NCT01225640.
Conflict of interest statement
Figures

References
-
- World Health Organization (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 Global report on surveillance and response. WHO/HTM/TB/2010.3.
-
- Williams KN, Brickner SJ, Stover CK, Zhu T, Ogden A, et al. (2009) Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med 180: 371–376. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

