Dietary triglycerides act on mesolimbic structures to regulate the rewarding and motivational aspects of feeding
- PMID: 24732670
- PMCID: PMC4303340
- DOI: 10.1038/mp.2014.31
Dietary triglycerides act on mesolimbic structures to regulate the rewarding and motivational aspects of feeding
Abstract
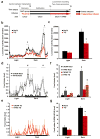
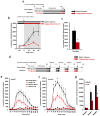
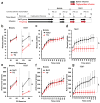
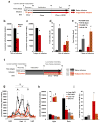
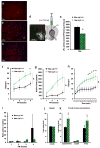
Circulating triglycerides (TGs) normally increase after a meal but are altered in pathophysiological conditions, such as obesity. Although TG metabolism in the brain remains poorly understood, several brain structures express enzymes that process TG-enriched particles, including mesolimbic structures. For this reason, and because consumption of high-fat diet alters dopamine signaling, we tested the hypothesis that TG might directly target mesolimbic reward circuits to control reward-seeking behaviors. We found that the delivery of small amounts of TG to the brain through the carotid artery rapidly reduced both spontaneous and amphetamine-induced locomotion, abolished preference for palatable food and reduced the motivation to engage in food-seeking behavior. Conversely, targeted disruption of the TG-hydrolyzing enzyme lipoprotein lipase specifically in the nucleus accumbens increased palatable food preference and food-seeking behavior. Finally, prolonged TG perfusion resulted in a return to normal palatable food preference despite continued locomotor suppression, suggesting that adaptive mechanisms occur. These findings reveal new mechanisms by which dietary fat may alter mesolimbic circuit function and reward seeking.
Conflict of interest statement
The authors declared that no conflict of interest exist
Figures
References
-
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. - PubMed
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. - PubMed
-
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81(5):781–793. - PubMed
-
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

