Mechanism of action of phenethylisothiocyanate and other reactive oxygen species-inducing anticancer agents
- PMID: 24732804
- PMCID: PMC4054319
- DOI: 10.1128/MCB.01602-13
Mechanism of action of phenethylisothiocyanate and other reactive oxygen species-inducing anticancer agents
Abstract
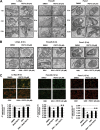
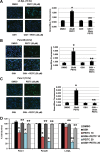
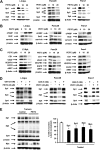
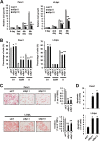
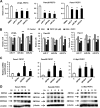
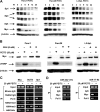
Reactive oxygen species (ROS)-inducing anticancer agents such as phenethylisothiocyanate (PEITC) activate stress pathways for killing cancer cells. Here we demonstrate that PEITC-induced ROS decreased expression of microRNA 27a (miR-27a)/miR-20a:miR-17-5p and induced miR-regulated ZBTB10/ZBTB4 and ZBTB34 transcriptional repressors, which, in turn, downregulate specificity protein (Sp) transcription factors (TFs) Sp1, Sp3, and Sp4 in pancreatic cancer cells. Decreased expression of miR-27a/miR-20a:miR-17-5p by PEITC-induced ROS is a key step in triggering the miR-ZBTB Sp cascade leading to downregulation of Sp TFs, and this is due to ROS-dependent epigenetic effects associated with genome-wide shifts in repressor complexes, resulting in decreased expression of Myc and the Myc-regulated miRs. Knockdown of Sp1 alone by RNA interference also induced apoptosis and decreased pancreatic cancer cell growth and invasion, indicating that downregulation of Sp transcription factors is an important common mechanism of action for PEITC and other ROS-inducing anticancer agents.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
