The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism
- PMID: 24732809
- PMCID: PMC4195816
- DOI: 10.1016/j.canlet.2014.04.001
The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism
Abstract
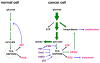
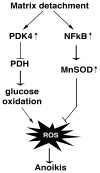
Compared to normal cells, cancer cells strongly upregulate glucose uptake and glycolysis to give rise to increased yield of intermediate glycolytic metabolites and the end product pyruvate. Moreover, glycolysis is uncoupled from the mitochondrial tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) in cancer cells. Consequently, the majority of glycolysis-derived pyruvate is diverted to lactate fermentation and kept away from mitochondrial oxidative metabolism. This metabolic phenotype is known as the Warburg effect. While it has become widely accepted that the glycolytic intermediates provide essential anabolic support for cell proliferation and tumor growth, it remains largely elusive whether and how the Warburg metabolic phenotype may play a role in tumor progression. We hereby review the cause and consequence of the restrained oxidative metabolism, in particular in the context of tumor metastasis. Cells change or lose their extracellular matrix during the metastatic process. Inadequate/inappropriate matrix attachment generates reactive oxygen species (ROS) and causes a specific type of cell death, termed anoikis, in normal cells. Although anoikis is a barrier to metastasis, cancer cells have often acquired elevated threshold for anoikis and hence heightened metastatic potential. As ROS are inherent byproducts of oxidative metabolism, forced stimulation of glucose oxidation in cancer cells raises oxidative stress and restores cells' sensitivity to anoikis. Therefore, by limiting the pyruvate flux into mitochondrial oxidative metabolism, the Warburg effect enables cancer cells to avoid excess ROS generation from mitochondrial respiration and thus gain increased anoikis resistance and survival advantage for metastasis. Consistent with this notion, pro-metastatic transcription factors HIF and Snail attenuate oxidative metabolism, whereas tumor suppressor p53 and metastasis suppressor KISS1 promote mitochondrial oxidation. Collectively, these findings reveal mitochondrial oxidative metabolism as a critical suppressor of metastasis and justify metabolic therapies for potential prevention/intervention of tumor metastasis.
Keywords: Anoikis; Glycolysis; Metastasis; OXPHOS; ROS; Warburg effect.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
None
Figures

References
-
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008 Jun;13(6):472–82. - PubMed
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008 Sep;134(5):703–7. - PubMed
-
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011 May;11(5):325–37. - PubMed
-
- Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011 Jun;1807(6):552–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

