Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies
- PMID: 24732893
- PMCID: PMC3986240
- DOI: 10.1371/journal.pone.0094764
Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies
Abstract
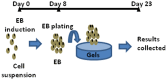
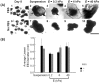
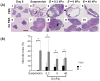
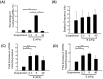
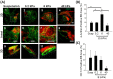
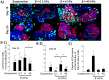
Stem cell clusters, such as embryoid bodies (EBs) derived from embryonic stem cells, are extensively studied for creation of multicellular clusters and complex functional tissues. It is common to control phenotypes of ES cells with varying molecular compounds; however, there is still a need to improve the controllability of cell differentiation, and thus, the quality of created tissue. This study demonstrates a simple but effective strategy to promote formation of vascularized cardiac muscle-like tissue in EBs and form contracting cardiovascular organoids by modulating the stiffness of a cell adherent hydrogel. Using collagen-conjugated polyacrylamide hydrogels with controlled elastic moduli, we discovered that cellular organization in a form of vascularized cardiac muscle sheet was maximal on the gel with the stiffness similar to cardiac muscle. We envisage that the results of this study will greatly contribute to better understanding of emergent behavior of stem cells in developmental and regeneration process and will also expedite translation of EB studies to drug-screening device assembly and clinical treatments.
Conflict of interest statement
Figures
References
-
- Carpenedo RL, Sargent CY, McDevitt TC (2007) Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 25: 2224–2234. - PubMed
-
- Liang Y, Jeong J, DeVolder RJ, Cha C, Wang F, et al. (2011) A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials 32: 9308–9315. - PubMed
-
- Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, et al. (1997) Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 29: 1525–1539. - PubMed
-
- McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ (1982) Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 299: 165–167. - PubMed
-
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, et al. (2000) Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408: 92–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

