Expression of DNAJB12 or DNAJB14 causes coordinate invasion of the nucleus by membranes associated with a novel nuclear pore structure
- PMID: 24732912
- PMCID: PMC3986390
- DOI: 10.1371/journal.pone.0094322
Expression of DNAJB12 or DNAJB14 causes coordinate invasion of the nucleus by membranes associated with a novel nuclear pore structure
Abstract
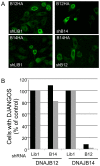
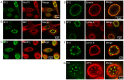
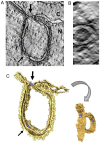
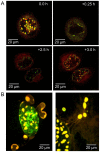
DNAJB12 and DNAJB14 are transmembrane proteins in the endoplasmic reticulum (ER) that serve as co-chaperones for Hsc70/Hsp70 heat shock proteins. We demonstrate that over-expression of DNAJB12 or DNAJB14 causes the formation of elaborate membranous structures within cell nuclei, which we designate DJANGOS for DNAJ-associated nuclear globular structures. DJANGOS contain DNAJB12, DNAJB14, Hsc70 and markers of the ER lumen and ER and nuclear membranes. Strikingly, they are evenly distributed underneath the nuclear envelope and are of uniform size in any one nucleus. DJANGOS are composed primarily of single-walled membrane tubes and sheets that connect to the nuclear envelope via a unique configuration of membranes, in which the nuclear pore complex appears anchored exclusively to the outer nuclear membrane, allowing both the inner and outer nuclear membranes to flow past the circumference of the nuclear pore complex into the nucleus. DJANGOS break down rapidly during cell division and reform synchronously in the daughter cell nuclei, demonstrating that they are dynamic structures that undergo coordinate formation and dissolution. Genetic studies showed that the chaperone activity of DNAJ/Hsc70 is required for the formation of DJANGOS. Further analysis of these structures will provide insight into nuclear pore formation and function, activities of molecular chaperones, and mechanisms that maintain membrane identity.
Conflict of interest statement
Figures



Similar articles
-
Cytosolic and endoplasmic reticulum chaperones inhibit wt-p53 to increase cancer cells' survival by refluxing ER-proteins to the cytosol.Elife. 2025 Apr 9;14:e102658. doi: 10.7554/eLife.102658. Elife. 2025. PMID: 40202782 Free PMC article.
-
A novel mammalian ER-located J-protein, DNAJB14, can accelerate ERAD of misfolded membrane proteins.Cell Struct Funct. 2012;37(2):177-87. doi: 10.1247/csf.12017. Epub 2012 Sep 27. Cell Struct Funct. 2012. PMID: 23018488
-
Novel functions of the ER-located Hsp40s DNAJB12 and DNAJB14 on proteins at the outer mitochondrial membrane under stress mediated by CCCP.Mol Cell Biochem. 2024 Oct;479(10):2637-2652. doi: 10.1007/s11010-023-04866-1. Epub 2023 Oct 18. Mol Cell Biochem. 2024. PMID: 37851175
-
Structural analysis of the nuclear pore complex by integrated approaches.Curr Opin Struct Biol. 2009 Apr;19(2):226-32. doi: 10.1016/j.sbi.2009.02.009. Epub 2009 Mar 25. Curr Opin Struct Biol. 2009. PMID: 19327984 Review.
-
Building a nuclear envelope at the end of mitosis: coordinating membrane reorganization, nuclear pore complex assembly, and chromatin de-condensation.Chromosoma. 2012 Dec;121(6):539-54. doi: 10.1007/s00412-012-0388-3. Epub 2012 Oct 27. Chromosoma. 2012. PMID: 23104094 Free PMC article. Review.
Cited by
-
Ubiquitin-interacting motifs of ataxin-3 regulate its polyglutamine toxicity through Hsc70-4-dependent aggregation.Elife. 2020 Sep 21;9:e60742. doi: 10.7554/eLife.60742. Elife. 2020. PMID: 32955441 Free PMC article.
-
Cytosolic and endoplasmic reticulum chaperones inhibit wt-p53 to increase cancer cells' survival by refluxing ER-proteins to the cytosol.Elife. 2025 Apr 9;14:e102658. doi: 10.7554/eLife.102658. Elife. 2025. PMID: 40202782 Free PMC article.
-
Differential roles for DNAJ isoforms in HTT-polyQ and FUS aggregation modulation revealed by chaperone screens.Nat Commun. 2022 Jan 26;13(1):516. doi: 10.1038/s41467-022-27982-w. Nat Commun. 2022. PMID: 35082301 Free PMC article.
-
Shared and Distinctive Neighborhoods of Emerin and Lamin B Receptor Revealed by Proximity Labeling and Quantitative Proteomics.J Proteome Res. 2022 Sep 2;21(9):2197-2210. doi: 10.1021/acs.jproteome.2c00281. Epub 2022 Aug 16. J Proteome Res. 2022. PMID: 35972904 Free PMC article.
-
Deciphering the Pharmacological Mechanism of the Herb Radix Ophiopogonis in the Treatment of Nasopharyngeal Carcinoma by Integrating iTRAQ-Coupled 2-D LC-MS/MS Analysis and Network Investigation.Front Pharmacol. 2019 Mar 18;10:253. doi: 10.3389/fphar.2019.00253. eCollection 2019. Front Pharmacol. 2019. PMID: 30936832 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

