Chlamydia trachomatis polymorphic membrane protein D is a virulence factor involved in early host-cell interactions
- PMID: 24733093
- PMCID: PMC4097629
- DOI: 10.1128/IAI.01686-14
Chlamydia trachomatis polymorphic membrane protein D is a virulence factor involved in early host-cell interactions
Abstract
Chlamydia trachomatis is an obligate intracellular mucosotropic pathogen of significant medical importance. It is the etiological agent of blinding trachoma and bacterial sexually transmitted diseases, infections that afflict hundreds of millions of people globally. The C. trachomatis polymorphic membrane protein D (PmpD) is a highly conserved autotransporter and the target of broadly cross-reactive neutralizing antibodies; however, its role in host-pathogen interactions is unknown. Here we employed a targeted reverse genetics approach to generate a pmpD null mutant that was used to define the role of PmpD in the pathogenesis of chlamydial infection. We show that pmpD is not an essential chlamydial gene and the pmpD null mutant has no detectable deficiency in cultured murine cells or in a murine mucosal infection model. Notably, however, the pmpD null mutant was significantly attenuated for macaque eyes and cultured human cells. A reduction in pmpD null infection of human endocervical cells was associated with a deficiency in chlamydial attachment to cells. Collectively, our results show that PmpD is a chlamydial virulence factor that functions in early host-cell interactions. This study is the first of its kind using reverse genetics to evaluate the contribution of a C. trachomatis gene to disease pathogenesis.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

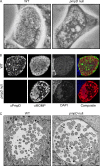
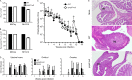
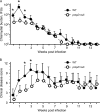

References
-
- World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. World Health Organization, Geneva, Switzerland
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

