An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production
- PMID: 24733347
- PMCID: PMC3986368
- DOI: 10.1371/journal.pone.0094997
An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production
Abstract
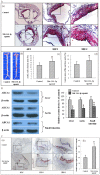
Aims: ATP-binding cassette transporter A1 (ABCA1) mediates the efflux of cholesterol and phospholipids to lipid-poor apolipoproteins, which then form nascent HDL, a key step in the mechanism of reverse cholesterol transport (RCT). While a series of microRNAs (miRNAs) have been identified as potent post-transcriptional regulators of lipid metabolism, their effects on ABCA1 function and associated mechanisms remain unclear.
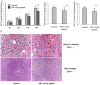
Methods and results: ABCA1 was identified as a potential target of miR-144-3p, based on the results of bioinformatic analysis and the luciferase reporter assay, and downregulated after transfection of cells with miR-144-3p mimics, as observed with real-time PCR and western blot. Moreover, miR-144-3p mimics (agomir) enhanced the expression of inflammatory factors, including IL-1β, IL-6 and TNF-α, in vivo and in vitro, inhibited cholesterol efflux in THP-1 macrophage-derived foam cells, decreased HDL-C circulation and impaired RCT in vivo, resulting in accelerated pathological progression of atherosclerosis in apoE-/- mice. Clinical studies additionally revealed a positive correlation of circulating miR-144-3p with serum CK, CK-MB, LDH and AST in subjects with AMI.
Conclusions: Our findings clearly indicate that miR-144-3p is essential for the regulation of cholesterol homeostasis and inflammatory reactions, supporting its utility as a potential therapeutic target of atherosclerosis and a promising diagnostic biomarker of AMI.
Conflict of interest statement
Figures

References
-
- Hansson GK (2009) Atherosclerosis–an immune disease: The Anitschkov Lecture 2007. Atherosclerosis 202: 2–10. - PubMed
-
- Dean M, Hamon Y, Chimini G (2001) The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res 42: 1007–1017. - PubMed
-
- Oram JF, Lawn RM, Garvin MR, Wade DP (2000) ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem 275: 34508–34511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

