Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog
- PMID: 24733352
- PMCID: PMC3986229
- DOI: 10.1371/journal.pone.0094732
Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog
Abstract
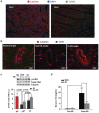
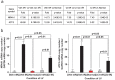
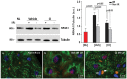
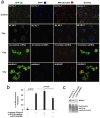
Cardiac ischemia and reperfusion (I/R) injury occurs because the acute increase in oxidative/inflammatory stress during reperfusion culminates in the death of cardiomyocytes. Currently, there is no drug utilized clinically that attenuates I/R injury in patients. Previous studies have demonstrated degranulation of mast cell contents into the interstitium after I/R. Using a dog model of I/R, we tested the role of chymase, a mast cell protease, in cardiomyocyte injury using a specific oral chymase inhibitor (CI). 15 adult mongrel dogs had left anterior descending artery occlusion for 60 min and reperfusion for 100 minutes. 9 dogs received vehicle and 6 were pretreated with a specific CI. In vivo cardiac microdialysis demonstrated a 3-fold increase in interstitial fluid chymase activity in I/R region that was significantly decreased by CI. CI pretreatment significantly attenuated loss of laminin, focal adhesion complex disruption, and release of troponin I into the circulation. Microarray analysis identified an I/R induced 17-fold increase in nuclear receptor subfamily 4A1 (NR4A1) and significantly decreased by CI. NR4A1 normally resides in the nucleus but can induce cell death on migration to the cytoplasm. I/R caused significant increase in NR4A1 protein expression and cytoplasmic translocation, and mitochondrial degradation, which were decreased by CI. Immunohistochemistry also revealed a high concentration of chymase within cardiomyocytes after I/R. In vitro, chymase added to culture HL-1 cardiomyocytes entered the cytoplasm and nucleus in a dynamin-dependent fashion, and promoted cytoplasmic translocation of NR4A1 protein. shRNA knockdown of NR4A1 on pre-treatment of HL-1 cells with CI significantly decreased chymase-induced cell death and mitochondrial damage. These results suggest that the beneficial effects of an orally active CI during I/R are mediated in the cardiac interstitium as well as within the cardiomyocyte due to a heretofore-unrecognized chymase entry into cardiomyocytes.
Conflict of interest statement
Figures





References
-
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury (2007) N Engl J Med. 357: 1121–1135. - PubMed
-
- Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, et al. (1998) Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation 98: 687–698. - PubMed
-
- Caughey GH, Raymond WW, Wolters PJ (2000) Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta 1480: 245–257. - PubMed
-
- Vartio T, Seppa H, Vaheri A (1981) Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 256: 471–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

