Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease
- PMID: 24733463
- PMCID: PMC4068540
- DOI: 10.1128/AAC.02686-13
Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease
Abstract
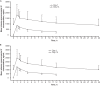
This was a phase 1B, dose-ranging, multicenter, pharmacokinetics, and safety study of cyclodextrin-based posaconazole intravenous (i.v.) solution administered through a central line to subjects at high risk for invasive fungal disease (part 1 of a 2-part study [phase 1B/3]). Initially, the safety and tolerability of single-dose posaconazole i.v. 200 mg (n = 10) were compared with those of a placebo (n = 11). Subsequently, 2 doses were evaluated, posaconazole i.v. 200 mg once daily (q.d.) (n = 21) and 300 mg q.d. (n = 24). The subjects received twice-daily (b.i.d.) posaconazole i.v. on day 1, followed by 13 days of posaconazole i.v. q.d., then 14 days of posaconazole oral suspension 400 mg b.i.d. The steady-state (day 14) exposure target (average concentration [areas under concentration-time curve {AUCs}/24 h, average concentrations at steady state {Cavgs}], of ≥ 500 to ≤ 2,500 ng/ml in ≥ 90% of the subjects) was achieved by 94% of the subjects for 200 mg posaconazole q.d. and by 95% of subjects for 300 mg posaconazole q.d. The desired exposure target (mean steady-state Cavg, ∼ 1,200 ng/ml) was 1,180 ng/ml in the 200-mg dosing cohort and was exceeded in the 300-mg dosing cohort (1,430 ng/ml). Posaconazole i.v. was well tolerated. Posaconazole i.v. 300 mg q.d. was selected for the phase 3 study segment. (This study has been registered at ClinicalTrials.gov under registration no. NCT01075984.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359. 10.1056/NEJMoa061094 - DOI - PubMed
-
- Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347. 10.1056/NEJMoa061098 - DOI - PubMed
-
- Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12. 10.1086/508774 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

