Lysine acetylation in sexual stage malaria parasites is a target for antimalarial small molecules
- PMID: 24733477
- PMCID: PMC4068603
- DOI: 10.1128/AAC.02721-13
Lysine acetylation in sexual stage malaria parasites is a target for antimalarial small molecules
Abstract
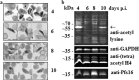
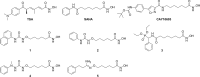
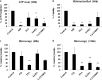
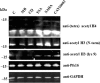
Therapies to prevent transmission of malaria parasites to the mosquito vector are a vital part of the global malaria elimination agenda. Primaquine is currently the only drug with such activity; however, its use is limited by side effects. The development of transmission-blocking strategies requires an understanding of sexual stage malaria parasite (gametocyte) biology and the identification of new drug leads. Lysine acetylation is an important posttranslational modification involved in regulating eukaryotic gene expression and other essential processes. Interfering with this process with histone deacetylase (HDAC) inhibitors is a validated strategy for cancer and other diseases, including asexual stage malaria parasites. Here we confirm the expression of at least one HDAC protein in Plasmodium falciparum gametocytes and show that histone and nonhistone protein acetylation occurs in this life cycle stage. The activity of the canonical HDAC inhibitors trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA; Vorinostat) and a panel of novel HDAC inhibitors on early/late-stage gametocytes and on gamete formation was examined. Several compounds displayed early/late-stage gametocytocidal activity, with TSA being the most potent (50% inhibitory concentration, 70 to 90 nM). In contrast, no inhibitory activity was observed in P. falciparum gametocyte exflagellation experiments. Gametocytocidal HDAC inhibitors caused hyperacetylation of gametocyte histones, consistent with a mode of action targeting HDAC activity. Our data identify HDAC inhibitors as being among a limited number of compounds that target both asexual and sexual stage malaria parasites, making them a potential new starting point for gametocytocidal drug leads and valuable tools for dissecting gametocyte biology.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. 10.1016/S0140-6736(12)60484-X - DOI - PMC - PubMed
-
- Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 9:136. 10.1186/1475-2875-9-136 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

