MuRF1 activity is present in cardiac mitochondria and regulates reactive oxygen species production in vivo
- PMID: 24733503
- PMCID: PMC4047114
- DOI: 10.1007/s10863-014-9549-9
MuRF1 activity is present in cardiac mitochondria and regulates reactive oxygen species production in vivo
Erratum in
-
Erratum to: MuRF1 activity is present in cardiac mitochondria and regulates reactive oxygen species production in vivo.J Bioenerg Biomembr. 2015 Jun;47(3):281. doi: 10.1007/s10863-014-9597-1. J Bioenerg Biomembr. 2015. PMID: 25515723 No abstract available.
Abstract
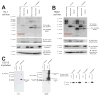
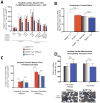
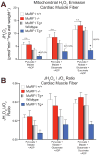
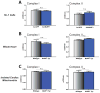
MuRF1 is a previously reported ubiquitin-ligase found in striated muscle that targets troponin I and myosin heavy chain for degradation. While MuRF1 has been reported to interact with mitochondrial substrates in yeast two-hybrid studies, no studies have identified MuRF1's role in regulating mitochondrial function to date. In the present study, we measured cardiac mitochondrial function from isolated permeabilized muscle fibers in previously phenotyped MuRF1 transgenic and MuRF1-/- mouse models to determine the role of MuRF1 in intermediate energy metabolism and ROS production. We identified a significant decrease in reactive oxygen species production in cardiac muscle fibers from MuRF1 transgenic mice with increased α-MHC driven MuRF1 expression. Increased MuRF1 expression in ex vivo and in vitro experiments revealed no alterations in the respiratory chain complex I and II function. Working perfusion experiments on MuRF1 transgenic hearts demonstrated significant changes in glucose oxidation. However, total oxygen consumption was decreased [corrected]. This data provides evidence for MuRF1 as a novel regulator of cardiac ROS, offering another mechanism by which increased MuRF1 expression may be cardioprotective in ischemia reperfusion injury, in addition to its inhibition of apoptosis via proteasome-mediate degradation of c-Jun. The lack of mitochondrial function phenotype identified in MuRF1-/- hearts may be due to the overlapping interactions of MuRF1 and MuRF2 with energy regulating proteins found by yeast two-hybrid studies reported here, implying a duplicity in MuRF1 and MuRF2's regulation of mitochondrial function.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures


References
-
- Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, et al. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] J Mol Biol. 2001;306(4):717–726. doi: 10.1006/jmbi.2001.4448. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

