New antiarrhythmic targets to control intracellular calcium handling
- PMID: 24733689
- PMCID: PMC4016334
- DOI: 10.1007/s12471-014-0549-5
New antiarrhythmic targets to control intracellular calcium handling
Abstract
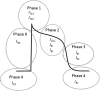
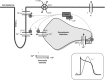
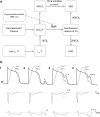
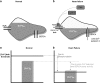
Sudden cardiac death due to ventricular arrhythmias is a major problem. Drug therapies to prevent SCD do not provide satisfying results, leading to the demand for new antiarrhythmic strategies. New targets include Ca(2+)/Calmodulin-dependent protein kinase II (CaMKII), the Na/Ca exchanger (NCX), the Ryanodine receptor (RyR, and its associated protein FKBP12.6 (Calstabin)) and the late component of the sodium current (I Na-Late ), all related to intracellular calcium (Ca(2+)) handling. In this review, drugs interfering with these targets (SEA-0400, K201, KN-93, W7, ranolazine, sophocarpine, and GS-967) are evaluated and their future as clinical compounds is considered. These new targets prove to be interesting; however more insight into long-term drug effects is necessary before clinical applicability becomes reality.
Figures

Similar articles
-
Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes.Free Radic Biol Med. 2013 Oct;63:338-49. doi: 10.1016/j.freeradbiomed.2013.05.035. Epub 2013 Jun 1. Free Radic Biol Med. 2013. PMID: 23732518 Review.
-
Ca2+/calmodulin-dependent kinase II-dependent regulation of atrial myocyte late Na+ current, Ca2+ cycling, and excitability: a mathematical modeling study.Am J Physiol Heart Circ Physiol. 2017 Dec 1;313(6):H1227-H1239. doi: 10.1152/ajpheart.00185.2017. Epub 2017 Aug 25. Am J Physiol Heart Circ Physiol. 2017. PMID: 28842436 Free PMC article.
-
Ranolazine prevents pressure overload-induced cardiac hypertrophy and heart failure by restoring aberrant Na+ and Ca2+ handling.J Cell Physiol. 2019 Jul;234(7):11587-11601. doi: 10.1002/jcp.27791. Epub 2018 Nov 29. J Cell Physiol. 2019. PMID: 30488495 Free PMC article.
-
Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice.Am J Physiol Cell Physiol. 2011 Sep;301(3):C577-86. doi: 10.1152/ajpcell.00125.2011. Epub 2011 Jun 15. Am J Physiol Cell Physiol. 2011. PMID: 21677263
-
Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death.Circ Res. 2015 Jun 5;116(12):1956-70. doi: 10.1161/CIRCRESAHA.116.304678. Circ Res. 2015. PMID: 26044250 Free PMC article. Review.
Cited by
-
The Arrhythmogenic Calmodulin p.Phe142Leu Mutation Impairs C-domain Ca2+ Binding but Not Calmodulin-dependent Inhibition of the Cardiac Ryanodine Receptor.J Biol Chem. 2017 Jan 27;292(4):1385-1395. doi: 10.1074/jbc.M116.766253. Epub 2016 Dec 7. J Biol Chem. 2017. PMID: 27927985 Free PMC article.
-
An Optogenetic Arrhythmia Model-Insertion of Several Catecholaminergic Polymorphic Ventricular Tachycardia Mutations Into Caenorhabditis elegans UNC-68 Disturbs Calstabin-Mediated Stabilization of the Ryanodine Receptor Homolog.Front Physiol. 2022 Mar 25;13:691829. doi: 10.3389/fphys.2022.691829. eCollection 2022. Front Physiol. 2022. PMID: 35399287 Free PMC article.
-
First Insight on Small Molecules as Cardiac Calsequestrin Stabilizers.ACS Omega. 2019 Jul 2;4(7):11508-11514. doi: 10.1021/acsomega.9b01113. eCollection 2019 Jul 31. ACS Omega. 2019. PMID: 31460256 Free PMC article.
-
Wenxin Keli for the Treatment of Arrhythmia-Systems Pharmacology and In Vivo Pharmacological Assessment.Front Pharmacol. 2021 Aug 26;12:704622. doi: 10.3389/fphar.2021.704622. eCollection 2021. Front Pharmacol. 2021. PMID: 34512338 Free PMC article.
-
A combined CaMKII inhibition and mineralocorticoid receptor antagonism via eplerenone inhibits functional deterioration in chronic pressure overloaded mice.J Cell Mol Med. 2020 Aug;24(15):8417-8429. doi: 10.1111/jcmm.15355. Epub 2020 Jun 23. J Cell Mol Med. 2020. PMID: 32573944 Free PMC article.
References
-
- Currie S, Elliott EB, Smith GL, et al. Two candidates at the heart of dysfunction: the ryanodine receptor and calcium/calmodulin protein kinase ii as potential targets for therapeutic intervention-an in vivo perspective. Pharmacol Ther. 2011;131:204–220. doi: 10.1016/j.pharmthera.2011.02.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

