A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats
- PMID: 24733787
- PMCID: PMC4053993
- DOI: 10.1124/dmd.114.057034
A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats
Erratum in
-
Correction to "A Recombinant Humanized Anti-Cocaine Monoclonal Antibody Inhibits the Distribution of Cocaine to the Brain in Rats".Drug Metab Dispos. 2016 Sep;44(9):1460-2. doi: 10.1124/dmd.114.057034err. Drug Metab Dispos. 2016. PMID: 27481865 Free PMC article. No abstract available.
Abstract
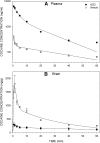
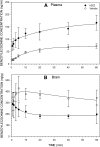
The monoclonal antibody (mAb), h2E2, is a humanized version of the chimeric human/murine anti-cocaine mAb 2E2. The recombinant h2E2 protein was produced in vitro from a transfected mammalian cell line and retained high affinity (4 nM Kd) and specificity for cocaine over its inactive metabolites benzoylecgonine (BE) and ecgonine methyl ester. In rats, pharmacokinetic studies of h2E2 (120 mg/kg i.v.) showed a long terminal elimination half-life of 9.0 days and a low volume of distribution at steady state (Vdss) of 0.3 l/kg. Pretreatment with h2E2 produced a dramatic 8.8-fold increase in the area under the plasma cocaine concentration-time curve (AUC) and in brain a concomitant decrease of 68% of cocaine's AUC following an i.v. injection of an equimolar cocaine dose. Sequestration of cocaine in plasma by h2E2, shown via reduction of cocaine's Vdss, indicates potential clinical efficacy. Although the binding of cocaine to h2E2 in plasma should inhibit distribution and metabolism, the elimination of cocaine remained multicompartmental and was still rapidly eliminated from plasma despite the presence of h2E2. BE was the major cocaine metabolite, and brain BE concentrations were sixfold higher than in plasma, indicating that cocaine is normally metabolized in the brain. In the presence of h2E2, brain BE concentrations were decreased and plasma BE was increased, consistent with the observed h2E2-induced changes in cocaine disposition. The inhibition of cocaine distribution to the brain confirms the humanized mAb, h2E2, as a lead candidate for development as an immunotherapy for cocaine abuse.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Bazin-Redureau MI, Renard CB, Scherrmann JM. (1997) Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab′)2 and Fab after intravenous administration in the rat. J Pharm Pharmacol 49:277–281 - PubMed
-
- Bleck GT. (2012) Consistent production of genetically stable mammalian cell lines. BioPharm International 25:56–59
-
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. (1995) Suppression of psychoactive effects of cocaine by active immunization. Nature 378:727–730 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

