Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: a phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance])
- PMID: 24733799
- PMCID: PMC4026580
- DOI: 10.1200/JCO.2013.53.2630
Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: a phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance])
Abstract
Purpose: Painful oral mucositis (OM) is a significant toxicity during radiotherapy for head and neck cancers. The aim of this randomized, double-blind, placebo-controlled trial was to test the efficacy of doxepin hydrochloride in the reduction of radiotherapy-induced OM pain.
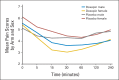
Patients and methods: In all, 155 patients were randomly allocated to a doxepin oral rinse or a placebo for the treatment of radiotherapy-related OM pain. Patients received a single dose of doxepin or placebo on day 1 and then crossed over to receive the opposite agent on a subsequent day. Pain questionnaires were administered at baseline and at 5, 15, 30, 60, 120, and 240 minutes. Patients were then given the option to continue doxepin. The primary end point was pain reduction as measured by the area under the curve (AUC) of the pain scale using data from day 1.
Results: Primary end point analysis revealed that the AUC for mouth and throat pain reduction was greater for doxepin (-9.1) than for placebo (-4.7; P < .001). Crossover analysis of patients completing both phases confirmed that patients experienced greater mouth and throat pain reduction with doxepin (intrapatient changes of 4.1 for doxepin-placebo arm and -2.8 for placebo-doxepin arm; P < .001). Doxepin was associated with more stinging or burning, unpleasant taste, and greater drowsiness than the placebo rinse. More patients receiving doxepin expressed a desire to continue treatment than did patients with placebo after completion of each of the randomized phases of the study.
Conclusion: A doxepin rinse diminishes OM pain. Further studies are warranted to determine its role in the management of OM.
Trial registration: ClinicalTrials.gov NCT01156142.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures




References
-
- Saadeh CE. Chemotherapy- and radiotherapy-induced oral mucositis: Review of preventive strategies and treatment. Pharmacotherapy. 2005;25:540–554. - PubMed
-
- Wong PC, Dodd MJ, Miaskowski C, et al. Mucositis pain induced by radiation therapy: Prevalence, severity, and use of self-care behaviors. J Pain Symptom Manage. 2006;32:27–37. - PubMed
-
- Elting LS, Cooksley CD, Chambers MS, et al. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110–1120. - PubMed
-
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

