Maturation of innate responses to mycobacteria over the first nine months of life
- PMID: 24733845
- PMCID: PMC4048703
- DOI: 10.4049/jimmunol.1400062
Maturation of innate responses to mycobacteria over the first nine months of life
Abstract
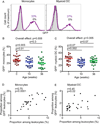
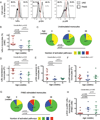
Newborns and young infants are particularly susceptible to infections, including Mycobacterium tuberculosis. Further, immunogenicity of vaccines against tuberculosis and other infectious diseases appears suboptimal early in life compared with later in life. We hypothesized that developmental changes in innate immunity would underlie these observations. To determine the evolution of innate responses to mycobacteria early in life, whole blood or PBMC from newborns, as well as 10- and 36-wk-old infants, was incubated with viable Mycobacterium bovis bacillus Calmette-Guérin or TLR ligands. Innate cell expression of cytokines and maturation markers was assessed, as well as activation of the proinflammatory NF-κB- and MAPK-signaling pathways. Bacillus Calmette-Guérin-induced production of the proinflammatory cytokines TNF-α, IL-6, and IL-12p40 increased from the newborn period to 9 mo of age in monocytes but not in myeloid dendritic cells. No changes in production of anti-inflammatory IL-10 were observed. CD40 expression increased with age in both cell populations. Older infants displayed substantial activation of all three signal transduction molecules: degradation of NF-κB inhibitor IκBα and phosphorylation of MAPK Erk and p38 upon TLR1/2 triggering, compared with predominant activation of only one of any of these molecules in newborns. Maturation of innate proinflammatory responses during the first 9 mo of life may underlie more effective control of mycobacteria and other pathogens observed later in infancy and age-related differential induction of Th1 responses by vaccination.
Conflict of interest statement
Figures

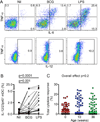
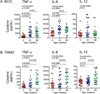
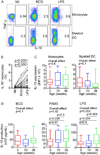
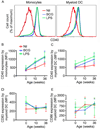
References
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Publishing Group. 2010;11:373–384. - PubMed
-
- Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine. 2007;13:460–469. - PubMed
-
- Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003;5:133–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

