Transcription factors TFIIF and TFIIS promote transcript elongation by RNA polymerase II by synergistic and independent mechanisms
- PMID: 24733897
- PMCID: PMC4020062
- DOI: 10.1073/pnas.1405181111
Transcription factors TFIIF and TFIIS promote transcript elongation by RNA polymerase II by synergistic and independent mechanisms
Abstract
Recent evidence suggests that transcript elongation by RNA polymerase II (RNAPII) is regulated by mechanical cues affecting the entry into, and exit from, transcriptionally inactive states, including pausing and arrest. We present a single-molecule optical-trapping study of the interactions of RNAPII with transcription elongation factors TFIIS and TFIIF, which affect these processes. By monitoring the response of elongation complexes containing RNAPII and combinations of TFIIF and TFIIS to controlled mechanical loads, we find that both transcription factors are independently capable of restoring arrested RNAPII to productive elongation. TFIIS, in addition to its established role in promoting transcript cleavage, is found to relieve arrest by a second, cleavage-independent mechanism. TFIIF synergistically enhances some, but not all, of the activities of TFIIS. These studies also uncovered unexpected insights into the mechanisms underlying transient pauses. The direct visualization of pauses at near-base-pair resolution, together with the load dependence of the pause-entry phase, suggests that two distinct mechanisms may be at play: backtracking under forces that hinder transcription and a backtrack-independent activity under assisting loads. The measured pause lifetime distributions are inconsistent with prevailing views of backtracking as a purely diffusive process, suggesting instead that the extent of backtracking may be modulated by mechanisms intrinsic to RNAPII. Pauses triggered by inosine triphosphate misincorporation led to backtracking, even under assisting loads, and their lifetimes were reduced by TFIIS, particularly when aided by TFIIF. Overall, these experiments provide additional insights into how obstacles to transcription may be overcome by the concerted actions of multiple accessory factors.
Keywords: Pol II; optical trap; optical tweezers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

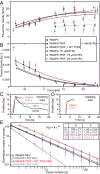
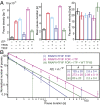
References
-
- Selth LA, Sigurdsson S, Svejstrup JQ. Transcript elongation by RNA polymerase II. Annu Rev Biochem. 2010;79:271–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

