Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity
- PMID: 24733916
- PMCID: PMC4035966
- DOI: 10.1073/pnas.1321507111
Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity
Abstract
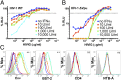
Tetherin is an IFN-inducible transmembrane protein that inhibits the detachment of enveloped viruses from infected cells. HIV-1 overcomes this restriction factor by expressing HIV-1 viral protein U (Vpu), which down-regulates and degrades tetherin. We report that mutations in Vpu that impair tetherin antagonism increase the susceptibility of HIV-infected cells to antibody-dependent cell-mediated cytotoxicity (ADCC), and conversely that RNAi knockdown of tetherin, but not other cellular proteins down-modulated by Vpu, decreases the susceptibility of HIV-infected cells to ADCC. These results reveal that Vpu protects HIV-infected cells from ADCC as a function of its ability to counteract tetherin. By serving as link between innate and adaptive immunity, the antiviral activity of tetherin may be augmented by virus-specific antibodies, and hence much greater than previously appreciated.
Keywords: AIDS; BST-2; CD317; lentivirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kupzig S, et al. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4(10):694–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

