Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake
- PMID: 24733919
- PMCID: PMC4035970
- DOI: 10.1073/pnas.1320421111
Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake
Abstract
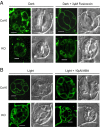
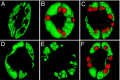
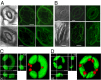
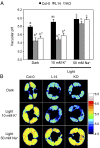
Stomatal movements rely on alterations in guard cell turgor. This requires massive K(+) bidirectional fluxes across the plasma and tonoplast membranes. Surprisingly, given their physiological importance, the transporters mediating the energetically uphill transport of K(+) into the vacuole remain to be identified. Here, we report that, in Arabidopsis guard cells, the tonoplast-localized K(+)/H(+) exchangers NHX1 and NHX2 are pivotal in the vacuolar accumulation of K(+) and that nhx1 nhx2 mutant lines are dysfunctional in stomatal regulation. Hypomorphic and complete-loss-of-function double mutants exhibited significantly impaired stomatal opening and closure responses. Disruption of K(+) accumulation in guard cells correlated with more acidic vacuoles and the disappearance of the highly dynamic remodelling of vacuolar structure associated with stomatal movements. Our results show that guard cell vacuolar accumulation of K(+) is a requirement for stomatal opening and a critical component in the overall K(+) homeostasis essential for stomatal closure, and suggest that vacuolar K(+) fluxes are also of decisive importance in the regulation of vacuolar dynamics and luminal pH that underlie stomatal movements.
Keywords: luminal pH control; stomata.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
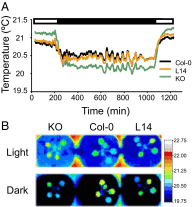
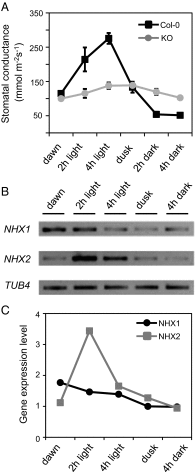
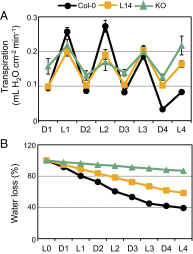
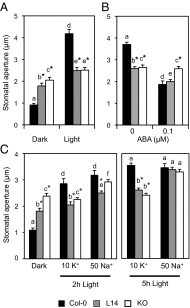
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

