The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro
- PMID: 24733958
- PMCID: PMC3964838
- DOI: 10.1155/2014/105420
The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro
Abstract
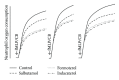
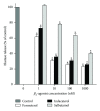
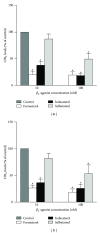
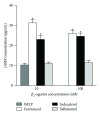
The clinical relevance of the anti-inflammatory properties of beta-2 agonists remains contentious possibly due to differences in their molecular structures and agonist activities. The current study has compared the effects of 3 different categories of β 2-agonists, namely, salbutamol (short-acting), formoterol (long-acting) and indacaterol (ultra-long-acting), at concentrations of 1-1000 nM, with human blood neutrophils in vitro. Neutrophils were activated with either N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP, 1 µM) or platelet-activating factor (PAF, 200 nM) in the absence and presence of the β 2-agonists followed by measurement of the generation of reactive oxygen species and leukotriene B4, release of elastase, and expression of the β 2-integrin, CR3, using a combination of chemiluminescence, ELISA, colorimetric, and flow cytometric procedures respectively. These were correlated with alterations in the concentrations of intracellular cyclic-AMP and cytosolic Ca(2+). At the concentrations tested, formoterol and indacaterol caused equivalent, significant (P < 0.05 at 1-10 nM) dose-related inhibition of all of the pro-inflammatory activities tested, while salbutamol was much less effective (P < 0.05 at 100 nM and higher). Suppression of neutrophil reactivity was accompanied by elevations in intracellular cAMP and accelerated clearance of Ca(2+) from the cytosol of activated neutrophils. These findings demonstrate that β 2-agonists vary with respect to their suppressive effects on activated neutrophils.
Figures


References
-
- Cazzola M, Page CP, Rogliani P, Matera MG. β-agonist therapy in lung disease. American Journal of Respiratory and Critical Care Medicine. 2013;187(7):690–696. - PubMed
-
- Sears MR. The FDA-mandated trial of safety of long-acting beta-agonists in asthma: finality or futility? Thorax. 2013;68(2):195–198. - PubMed
-
- Johnson M. Effects of β2-agonists on resident and infiltrating inflammatory cells. Journal of Allergy and Clinical Immunology. 2002;110(6):S282–S290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

