Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections
- PMID: 24733968
- PMCID: PMC3984887
- DOI: 10.31887/DCNS.2014.16.1/rduman
Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections
Abstract
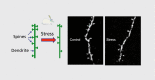
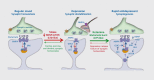
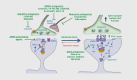
Despite the complexity and heterogeneity of mood disorders, basic and clinical research studies have begun to elucidate the pathophysiology of depression and to identify rapid, efficacious antidepressant agents. Stress and depression are associated with neuronal atrophy, characterized by loss of synaptic connections in key cortical and limbic brain regions implicated in depression. This is thought to occur in part via decreased expression and function of growth factors, such as brain-derived neurotrophic factor (BDNF), in the prefrontal cortex (PFC) and hippocampus. These structural alterations are difficult to reverse with typical antidepressants. However, recent studies demonstrate that ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist that produces rapid antidepressant actions in treatment-resistant depressed patients, rapidly increases spine synapses in the PFC and reverses the deficits caused by chronic stress. This is thought to occur by disinhibition of glutamate transmission, resulting in a rapid but transient burst of glutamate, followed by an increase in BDNF release and activation of downstream signaling pathways that stimulate synapse formation. Recent work demonstrates that the rapid-acting antidepressant effects of scopolamine, a muscarinic receptor antagonist, are also associated with increased glutamate transmission and synapse formation. These findings have resulted in testing and identification of additional targets and agents that influence glutamate transmission and have rapid antidepressant actions in rodent models and in clinical trials. Together these studies have created tremendous excitement and hope for a new generation of rapid, efficacious antidepressants.
A pesar de la complejidad y heterogeneidad de los trastornos del ánimo, los estudios de investigación básicos y clínicos han comenzado a aclarar la fisio patología de la depresión y a identificar agentes antidepresivos rápidos y eficaces. El estrés y la depresión están asociados con atrofia neural, caracterizada por pérdida de conexiones sinápticas en regiones corticales y Iímbicas claves que están implicadas en la depresión. Se cree que esto ocurre en parte a través de la reducción de la expresión y función de los factores de crecimiento, como el factor neurotrófico derivado del cerebro (BDNF) en la corteza prefrontal (CPF) y en el hipocampo. Estas alteraciones estructurales son difíciles de revertir con antidepresivos típicos. Sin embargo; estudios recientes demuestran que la ketamina, un antagonista del receptor N-metil-D-aspártico (NMDA) que produce rápidas acciones anti-depresivas en los pacientes con depresión resistente al tratamiento, aumenta rápidamente las espinas sinápticas en la CPF y revierte los déficits causados por el estrés crónico. Se cree que esto ocurre por desinhíbición de la transmisión glutamatérgica, lo que se traduce en un incremento rápido, pero transitorio de glutamato, seguido de un aumento de la liberación de BDNF y activación de las vías de señales hacia abajo que estimulan la formación de sinapsis. Un trabajo reciente demuestra que los efectos antidepresivos de rápida acción de la escopolamina, un antagonista del receptor muscarínico, también están asociados con un aumento de la transmisión glutamatérgíca y la formación de sinapsís. Estos hallazgos se han traducido en pruebas e identificación de objetivos adicionales y agentes que afectan la transmisión glutamatérgica, y tienen rápida acción antidepresiva en modelos de roedores y en ensayos clínicos. En conjunto estos estudios han generado grandes ilusiones y esperanzas para una nueva generación de antídepresivos rápidos y eficaces.
Malgré la complexité et l'hétérogénéité des troubles de l'humeur, des études de recherche clinique et fondamentale ont commencé à élucider la physiopathologie de la dépression et à identifier des antidépresseurs rapides et efficaces. Le stress et la dépression sont associés à une atrophie neuronale, caractérisée par la perte des connexions synaptiques dans les régions cérébrales limbiques et corticales impliquées dans la dépression, ce qui se manifeste en partie par une diminution de l'expression et de la fonction des facteurs de croissance, comme le facteur neurotrophique dérivé du cerveau (BDNF), dans le cortex préfrontal (CPF) et l'hippocampe. Ces altérations structurales sont difficiles à supprimer avec les antidépresseurs classiques. Cependant, d'après des études récentes, la kétamine, un antagoniste du récepteur du N-méthyl-D-aspartate (NMDA) qui induit une action antidépressive rapide chez des patients déprimés résistants au traitement, augmente rapidement la formation des synapses avec les épines dendritiques dans le CPF et s'oppose aux déficits causés par le stress chronique. Ce mécanisme intervient par désinhibition de la transmission du glutamate, aboutissant à sa stimulation rapide mais brève, suivie d'une augmentation de la libération du BDNF et d'une activation des voies de signalisation en aval, qui stimulent la formation des synapses. Un travail récent démontre que les effets antidépresseurs d'action rapide de la scopolamine, un antagoniste du récepteur muscarinique, s'associent également à une augmentation de la transmission du glutamate et de la formation des synapses. Ces résultats ont conduit à vérifier et identifier dans un modèle murin et dans des études cliniques, les produits et les cibles supplémentaires influant sur la transmission du glutamate et ayant une action antidépressive rapide. Ces études ont toutes suscité un espoir et une excitation considérables pour une nouvelle génération d'antidépresseurs rapides et efficaces.
Keywords: BDNF; glutamate; ketamine; mTORC1; prefrontal cortex; rapamycin; scopolamine; stress; synaptogenesis.
Figures
References
-
- Trivedi M., Rush AJ., Wisniewski SR., et aI STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. - PubMed
-
- Duman R., Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
