Recovering stimulus locations using populations of eye-position modulated neurons in dorsal and ventral visual streams of non-human primates
- PMID: 24734008
- PMCID: PMC3975102
- DOI: 10.3389/fnint.2014.00028
Recovering stimulus locations using populations of eye-position modulated neurons in dorsal and ventral visual streams of non-human primates
Abstract
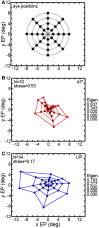
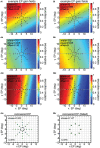
We recorded visual responses while monkeys fixated the same target at different gaze angles, both dorsally (lateral intraparietal cortex, LIP) and ventrally (anterior inferotemporal cortex, AIT). While eye-position modulations occurred in both areas, they were both more frequent and stronger in LIP neurons. We used an intrinsic population decoding technique, multidimensional scaling (MDS), to recover eye positions, equivalent to recovering fixated target locations. We report that eye-position based visual space in LIP was more accurate (i.e., metric). Nevertheless, the AIT spatial representation remained largely topologically correct, perhaps indicative of a categorical spatial representation (i.e., a qualitative description such as "left of" or "above" as opposed to a quantitative, metrically precise description). Additionally, we developed a simple neural model of eye position signals and illustrate that differences in single cell characteristics can influence the ability to recover target position in a population of cells. We demonstrate for the first time that the ventral stream contains sufficient information for constructing an eye-position based spatial representation. Furthermore we demonstrate, in dorsal and ventral streams as well as modeling, that target locations can be extracted directly from eye position signals in cortical visual responses without computing coordinate transforms of visual space.
Keywords: active vision; monkey; multidimensional scaling; population coding; spatial vision.
Figures






Similar articles
-
Population coding of visual space: comparison of spatial representations in dorsal and ventral pathways.Front Comput Neurosci. 2011 Feb 1;4:159. doi: 10.3389/fncom.2010.00159. eCollection 2011. Front Comput Neurosci. 2011. PMID: 21344010 Free PMC article.
-
Characteristics of Eye-Position Gain Field Populations Determine Geometry of Visual Space.Front Integr Neurosci. 2016 Jan 20;9:72. doi: 10.3389/fnint.2015.00072. eCollection 2015. Front Integr Neurosci. 2016. PMID: 26834587 Free PMC article.
-
Spatial modulation of primate inferotemporal responses by eye position.PLoS One. 2008;3(10):e3492. doi: 10.1371/journal.pone.0003492. Epub 2008 Oct 23. PLoS One. 2008. PMID: 18946508 Free PMC article.
-
Oculocentric spatial representation in parietal cortex.Cereb Cortex. 1995 Sep-Oct;5(5):470-81. doi: 10.1093/cercor/5.5.470. Cereb Cortex. 1995. PMID: 8547793 Review.
-
Functional interactions between the macaque dorsal and ventral visual pathways during three-dimensional object vision.Cortex. 2018 Jan;98:218-227. doi: 10.1016/j.cortex.2017.01.021. Epub 2017 Feb 3. Cortex. 2018. PMID: 28258716 Review.
Cited by
-
Attention Effects on Neural Population Representations for Shape and Location Are Stronger in the Ventral than Dorsal Stream.eNeuro. 2018 Jun 5;5(2):ENEURO.0371-17.2018. doi: 10.1523/ENEURO.0371-17.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29876521 Free PMC article.
-
Two Visual Pathways in Primates Based on Sampling of Space: Exploitation and Exploration of Visual Information.Front Integr Neurosci. 2016 Nov 22;10:37. doi: 10.3389/fnint.2016.00037. eCollection 2016. Front Integr Neurosci. 2016. PMID: 27920670 Free PMC article.
-
The Organization and Operation of Inferior Temporal Cortex.Annu Rev Vis Sci. 2018 Sep 15;4:381-402. doi: 10.1146/annurev-vision-091517-034202. Epub 2018 Jul 30. Annu Rev Vis Sci. 2018. PMID: 30059648 Free PMC article. Review.
-
The Intersection between Ocular and Manual Motor Control: Eye-Hand Coordination in Acquired Brain Injury.Front Neurol. 2017 Jun 1;8:227. doi: 10.3389/fneur.2017.00227. eCollection 2017. Front Neurol. 2017. PMID: 28620341 Free PMC article. Review.
-
Sensorimotor Self-organization via Circular-Reactions.Front Neurorobot. 2021 Dec 13;15:658450. doi: 10.3389/fnbot.2021.658450. eCollection 2021. Front Neurorobot. 2021. PMID: 34966265 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

