Parkin and PINK1: much more than mitophagy
- PMID: 24735649
- PMCID: PMC4075431
- DOI: 10.1016/j.tins.2014.03.004
Parkin and PINK1: much more than mitophagy
Abstract
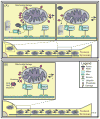
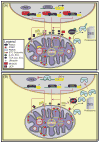
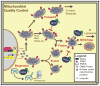
Parkinson's disease (PD) is a progressive neurodegenerative disease that causes a debilitating movement disorder. Although most cases of PD appear to be sporadic, rare Mendelian forms have provided tremendous insight into disease pathogenesis. Accumulating evidence suggests that impaired mitochondria underpin PD pathology. In support of this theory, data from multiple PD models have linked Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and parkin, two recessive PD genes, in a common pathway impacting mitochondrial health, prompting a flurry of research to identify their mitochondrial targets. Recent work has focused on the role of PINK1 and parkin in mediating mitochondrial autophagy (mitophagy); however, emerging evidence casts parkin and PINK1 as key players in multiple domains of mitochondrial health and quality control.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

