An enhancer composed of interlocking submodules controls transcriptional autoregulation of suppressor of hairless
- PMID: 24735880
- PMCID: PMC4013524
- DOI: 10.1016/j.devcel.2014.02.005
An enhancer composed of interlocking submodules controls transcriptional autoregulation of suppressor of hairless
Abstract
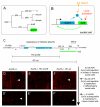
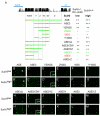
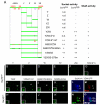
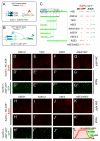
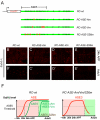
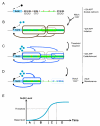
Positive autoregulation is an effective mechanism for the long-term maintenance of a transcription factor's expression. This strategy is widely deployed in cell lineages, where the autoregulatory factor controls the activity of a battery of genes that constitute the differentiation program of a postmitotic cell type. In Drosophila, the Notch pathway transcription factor Suppressor of Hairless activates its own expression, specifically in the socket cell of external sensory organs, via an autoregulatory enhancer called the ASE. Here, we show that the ASE is composed of several enhancer submodules, each of which can independently initiate weak Su(H) autoregulation. Cross-activation by these submodules is critical to ensure that Su(H) rises above a threshold level necessary to activate a maintenance submodule, which then sustains long-term Su(H) autoregulation. Our study reveals the use of interlinked positive-feedback loops to control autoregulation dynamically and provides mechanistic insight into initiation, establishment, and maintenance of the autoregulatory state.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Bailey AM, Posakony JW. Suppressor of Hairless directly activates transcription of Enhancer of split Complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. - PubMed
-
- Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW. A Notch-independent activity of Suppressor of Hairless is required for normal mechanoreceptor physiology. Cell. 2000;103:957–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

