Neuronostatin inhibits glucose-stimulated insulin secretion via direct action on the pancreatic α-cell
- PMID: 24735892
- PMCID: PMC4042099
- DOI: 10.1152/ajpendo.00599.2013
Neuronostatin inhibits glucose-stimulated insulin secretion via direct action on the pancreatic α-cell
Abstract
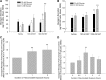
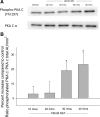
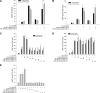
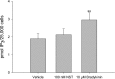
Neuronostatin is a recently described peptide hormone encoded by the somatostatin gene. We previously showed that intraperitoneal injection of neuronostatin into mice resulted in c-Jun accumulation in pancreatic islets in a pattern consistent with the activation of glucagon-producing α-cells. We therefore hypothesized that neuronostatin could influence glucose homeostasis via a direct effect on the α-cell. Neuronostatin enhanced low-glucose-induced glucagon release in isolated rat islets and in the immortalized α-cell line αTC1-9. Furthermore, incubation with neuronostatin led to an increase in transcription of glucagon mRNA, as determined by RT-PCR. Neuronostatin also inhibited glucose-stimulated insulin secretion from isolated islets. However, neuronostatin did not alter insulin release from the β-cell line INS 832/13, indicating that the effect of neuronostatin on insulin secretion may be secondary to a direct action on the α-cell. In agreement with our in vitro data, intra-arterial infusion of neuronostatin in male rats delayed glucose disposal and inhibited insulin release during a glucose challenge. These studies suggest that neuronostatin participates in maintaining glucose homeostasis through cell-cell interactions between α-cells and β-cells in the endocrine pancreas, leading to attenuation in insulin secretion.
Keywords: glucagon; glucose homeostasis; islet function; neuronostatin.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973 - PubMed
-
- Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes 57: 124–132, 2008 - PubMed
-
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007 - PubMed
-
- Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36: 391–392, 1974 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

