Safety and pharmacological characterization of the molecular tweezer CLR01 - a broad-spectrum inhibitor of amyloid proteins' toxicity
- PMID: 24735982
- PMCID: PMC3996151
- DOI: 10.1186/2050-6511-15-23
Safety and pharmacological characterization of the molecular tweezer CLR01 - a broad-spectrum inhibitor of amyloid proteins' toxicity
Abstract
Background: The "molecular tweezer" CLR01 is a broad-spectrum inhibitor of abnormal protein self-assembly, which acts by binding selectively to Lys residues. CLR01 has been tested in several in vitro and in vivo models of amyloidoses all without signs of toxicity. With the goal of developing CLR01 as a therapeutic drug for Alzheimer's disease and other amyloidoses, here we studied its safety and pharmacokinetics.
Methods: Toxicity studies were performed in 2-m old wild-type mice. Toxicity was evaluated by serum chemical analysis, histopathology analysis, and qualitative behavioral analysis. Brain penetration studies were performed using radiolabeled CLR01 in both wild-type mice and a transgenic mouse model of Alzheimer's disease at 2-m, 12-m, and 22-m of age. Brain levels were measured from 0.5 - 72 h post administration.
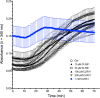
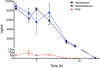
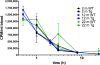
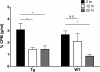
Results: Examination of CLR01's effect on tubulin polymerization, representing normal protein assembly, showed disruption of the process only when 55-fold excess CLR01 was used, supporting the compound's putative "process-specific" mechanism of action.A single-injection of 100 mg/kg CLR01 in mice - 2,500-fold higher than the efficacious dose reported previously, induced temporary distress and liver injury, but no mortality. Daily injection of doses up to 10 mg/kg did not produce any signs of toxicity, suggesting a high safety margin.The brain penetration of CLR01 was found to be 1 - 3% of blood levels depending on age. Though CLR01 was almost completely removed from the blood by 8 h, unexpectedly, brain levels of CLR01 remained steady over 72 h.
Conclusion: Estimation of brain levels compared to amyloid β-protein concentrations reported previously suggest that the stoichiometry obtained in vitro and in vivo is similar, supporting the mechanism of action of CLR01.The favorable safety margin of CLR01, together with efficacy shown in multiple animal models, support further development of CLR01 as a disease-modifying agent for amyloidoses.
Figures





References
-
- Highlights R. Chemical biology: aggravating aggregating. Nature. 2008;451:608–609.
-
- Roberts BE, Shorter J. Escaping amyloid fate. Nat Struct Mol Biol. 2008;15:544–546. - PubMed
-
- Sinha S, Lopes DH, Du Z, Pang ES, Shanmugam A, Lomakin A, Talbiersky P, Tennstaedt A, McDaniel K, Bakshi R, Kuo P-Y, Ehrmann M, Benedek GB, Loo JA, Klärner F-G, Schrader T, Wang C, Bitan G. Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins. J Am Chem Soc. 2011;133:16958–16969. - PMC - PubMed
-
- Attar A, Ripoli C, Riccardi E, Maiti P, Li Puma DD, Liu T, Hayes J, Jones MR, Lichti-Kaiser K, Yang F, Gale GD, Tseng C-H, Tan M, Xie C-W, Staudinger J-L, Klärner F-G, Schrader T, Frautschy SA, Grassi C, Bitan G. Protection of primary neurons and mouse brain from Alzheimer’s pathology by molecular tweezers. Brain. 2012;135:3735–3748. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

