Neural innervation of white adipose tissue and the control of lipolysis
- PMID: 24736043
- PMCID: PMC4175185
- DOI: 10.1016/j.yfrne.2014.04.001
Neural innervation of white adipose tissue and the control of lipolysis
Abstract
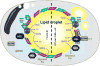
White adipose tissue (WAT) is innervated by the sympathetic nervous system (SNS) and its activation is necessary for lipolysis. WAT parasympathetic innervation is not supported. Fully-executed SNS-norepinephrine (NE)-mediated WAT lipolysis is dependent on β-adrenoceptor stimulation ultimately hinging on hormone sensitive lipase and perilipin A phosphorylation. WAT sympathetic drive is appropriately measured electrophysiologically and neurochemically (NE turnover) in non-human animals and this drive is fat pad-specific preventing generalizations among WAT depots and non-WAT organs. Leptin-triggered SNS-mediated lipolysis is weakly supported, whereas insulin or adenosine inhibition of SNS/NE-mediated lipolysis is strongly supported. In addition to lipolysis control, increases or decreases in WAT SNS drive/NE inhibit and stimulate white adipocyte proliferation, respectively. WAT sensory nerves are of spinal-origin and sensitive to local leptin and increases in sympathetic drive, the latter implicating lipolysis. Transsynaptic viral tract tracers revealed WAT central sympathetic and sensory circuits including SNS-sensory feedback loops that may control lipolysis.
Keywords: Denervation; Herpes simplex virus-1; Humans; Insulin; Leptin; Mice; Norepinephrine turnover; Pseudorabies virus; Rats; Siberian hamsters.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
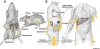
References
-
- Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. - PubMed
-
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. - PubMed
-
- Altuncu ME, Baspinar O, Keskin M. The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome. Cardiol J. 2012;19:501–506. - PubMed
-
- Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

