HIV-1 latency: an update of molecular mechanisms and therapeutic strategies
- PMID: 24736215
- PMCID: PMC4014718
- DOI: 10.3390/v6041715
HIV-1 latency: an update of molecular mechanisms and therapeutic strategies
Abstract
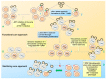
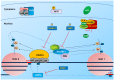
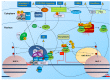
The major obstacle towards HIV-1 eradication is the life-long persistence of the virus in reservoirs of latently infected cells. In these cells the proviral DNA is integrated in the host's genome but it does not actively replicate, becoming invisible to the host immune system and unaffected by existing antiviral drugs. Rebound of viremia and recovery of systemic infection that follows interruption of therapy, necessitates life-long treatments with problems of compliance, toxicity, and untenable costs, especially in developing countries where the infection hits worst. Extensive research efforts have led to the proposal and preliminary testing of several anti-latency compounds, however, overall, eradication strategies have had, so far, limited clinical success while posing several risks for patients. This review will briefly summarize the more recent advances in the elucidation of mechanisms that regulates the establishment/maintenance of latency and therapeutic strategies currently under evaluation in order to eradicate HIV persistence.
Figures
References
-
- Barre-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C., et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. - PubMed
-
- Gallo R.C., Salahuddin S.Z., Popovic M., Shearer G.M., Kaplan M., Haynes B.F., Palker T.J., Redfield R., Oleske J., Safai B., et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. - PubMed
-
- Popovic M., Sarngadharan M.G., Read E., Gallo R.C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. - PubMed
-
- Sarngadharan M.G., Popovic M., Bruch L., Schupbach J., Gallo R.C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984;224:506–508. - PubMed
-
- Global report Unaids report on the global aids epidemic 2012. [(accessed on 1 December 2013)]. Available online: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

