A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa
- PMID: 24736222
- PMCID: PMC3993853
- DOI: 10.1128/mBio.00896-14
A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa
Abstract
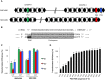
CRISPR-Cas systems are one of the most widespread phage resistance mechanisms in prokaryotes. Our lab recently identified the first examples of phage-borne anti-CRISPR genes that encode protein inhibitors of the type I-F CRISPR-Cas system of Pseudomonas aeruginosa. A key question arising from this work was whether there are other types of anti-CRISPR genes. In the current work, we address this question by demonstrating that some of the same phages carrying type I-F anti-CRISPR genes also possess genes that mediate inhibition of the type I-E CRISPR-Cas system of P. aeruginosa. We have discovered four distinct families of these type I-E anti-CRISPR genes. These genes do not inhibit the type I-F CRISPR-Cas system of P. aeruginosa or the type I-E system of Escherichia coli. Type I-E and I-F anti-CRISPR genes are located at the same position in the genomes of a large group of related P. aeruginosa phages, yet they are found in a variety of combinations and arrangements. We have also identified functional anti-CRISPR genes within nonprophage Pseudomonas genomic regions that are likely mobile genetic elements. This work emphasizes the potential importance of anti-CRISPR genes in phage evolution and lateral gene transfer and supports the hypothesis that more undiscovered families of anti-CRISPR genes exist. Finally, we provide the first demonstration that the type I-E CRISPR-Cas system of P. aeruginosa is naturally active without genetic manipulation, which contrasts with E. coli and other previously characterized I-E systems. IMPORTANCE The CRISPR-Cas system is an adaptive immune system possessed by the majority of prokaryotic organisms to combat potentially harmful foreign genetic elements. This study reports the discovery of bacteriophage-encoded anti-CRISPR genes that mediate inhibition of a well-studied subtype of CRISPR-Cas system. The four families of anti-CRISPR genes described here, which comprise only the second group of anti-CRISPR genes to be identified, encode small proteins that bear no sequence similarity to previously studied phage or bacterial proteins. Anti-CRISPR genes represent a newly discovered and intriguing facet of the ongoing evolutionary competition between phages and their bacterial hosts.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
