In vitro and in vivo studies of a rapid and selective breath test for tuberculosis based upon mycobacterial CO dehydrogenase
- PMID: 24736224
- PMCID: PMC3993857
- DOI: 10.1128/mBio.00990-14
In vitro and in vivo studies of a rapid and selective breath test for tuberculosis based upon mycobacterial CO dehydrogenase
Abstract
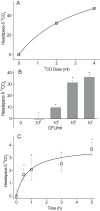
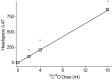
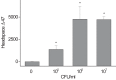
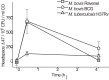
One of the major hurdles in treating tuberculosis (TB) is the time-consuming and difficult methodology for diagnosis. Stable-isotope breath tests hold great potential for rapidly diagnosing an infectious disease, monitoring therapy, and determining a bacterial phenotype in a rapid, point-of-care manner that does not require invasive sampling. Here we describe the preclinical development of a potentially highly selective TB diagnostic breath test based upon the organism's CO dehydrogenase activity. After development of the test in vitro, we were able to use the breath test to discriminate between infected and control rabbits, demonstrating that a diagnosis can potentially be made and also that a complex bacterial phenotype can be noninvasively and rapidly studied in the host. IMPORTANCE Tuberculosis (TB) remains a major infectious cause of disease and death worldwide, and effective diagnosis and then treatment are the tools with which we fight TB. The more quickly and more specific the diagnosis can be made, the better, and this is also true of diagnosis being as close to the patient (point of care) as possible. Here we report our preclinical development of breath tests based upon specific mycobacterial metabolism that could, with development, allow rapid point-of-care diagnosis through measuring the mycobacterial conversion of labeled CO to labeled CO2.
Figures
Similar articles
-
13[C]-urea breath test as a novel point-of-care biomarker for tuberculosis treatment and diagnosis.PLoS One. 2010 Aug 27;5(8):e12451. doi: 10.1371/journal.pone.0012451. PLoS One. 2010. PMID: 20805989 Free PMC article.
-
Current tuberculosis diagnostic tools & role of urease breath test.Indian J Med Res. 2012 May;135(5):731-6. Indian J Med Res. 2012. PMID: 22771606 Free PMC article. Review.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Reporter phage and breath tests: emerging phenotypic assays for diagnosing active tuberculosis, antibiotic resistance, and treatment efficacy.J Infect Dis. 2011 Nov 15;204 Suppl 4(Suppl 4):S1142-50. doi: 10.1093/infdis/jir454. J Infect Dis. 2011. PMID: 21996696 Free PMC article. Review.
-
Carbon monoxide dehydrogenase in mycobacteria possesses a nitric oxide dehydrogenase activity.Biochem Biophys Res Commun. 2007 Oct 19;362(2):449-53. doi: 10.1016/j.bbrc.2007.08.011. Epub 2007 Aug 10. Biochem Biophys Res Commun. 2007. PMID: 17707766
Cited by
-
Microbial oxidation of atmospheric trace gases.Nat Rev Microbiol. 2022 Sep;20(9):513-528. doi: 10.1038/s41579-022-00724-x. Epub 2022 Apr 12. Nat Rev Microbiol. 2022. PMID: 35414013 Review.
-
Biodiagnostics in an era of global pandemics-From biosensing materials to data management.View (Beijing). 2022 Mar;3(2):20200164. doi: 10.1002/VIW.20200164. Epub 2021 Jun 18. View (Beijing). 2022. PMID: 34766159 Free PMC article. Review.
-
A Review of Analytical Techniques and Their Application in Disease Diagnosis in Breathomics and Salivaomics Research.Int J Mol Sci. 2016 Dec 23;18(1):24. doi: 10.3390/ijms18010024. Int J Mol Sci. 2016. PMID: 28025547 Free PMC article. Review.
-
Rapid in vivo detection of isoniazid-sensitive Mycobacterium tuberculosis by breath test.Nat Commun. 2014 Sep 23;5:4989. doi: 10.1038/ncomms5989. Nat Commun. 2014. PMID: 25247851 Free PMC article.
-
Detecting virulence and drug-resistance mycobacterial phenotypes in vivo.Trends Microbiol. 2015 Jun;23(6):321-3. doi: 10.1016/j.tim.2015.02.013. Epub 2015 Mar 21. Trends Microbiol. 2015. PMID: 25800730 Free PMC article.
References
-
- Phillips PP, Gillespie SH, Boeree M, Heinrich N, Aarnoutse R, McHugh T, Pletschette M, Lienhardt C, Hafner R, Mgone C, Zumla A, Nunn AJ, Hoelscher M. 2012. Innovative trial designs are practical solutions for improving the treatment of tuberculosis. J. Infect. Dis. 205:S250–S257. 10.1093/infdis/jis041 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
